Igalmi
- Generic: dexmedetomidine
- Dosage Form sublingual film, for sublingual or buccal use
Medically reviewed by A Ras MD. Last updated April 8, 2022
What is Igalmi
IGALMI contains dexmedetomidine, an alpha2-adrenergic receptor agonist, present as dexmedetomidine hydrochloride, the S-enantiomer of medetomidine chemically described as 4-[(1S)-1(2, 3-dimethylphenyl) ethyl]-1H-imidazole hydrochloride. The empirical formula is C13H16N2•HCl with a molecular weight of 236.7 g/mol. The structural formula of dexmedetomidine hydrochloride is: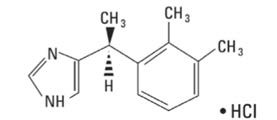
Dexmedetomidine hydrochloride is a white or almost white powder that is freely soluble in water and has a pKa of 7.1. Its partition coefficient in octanol/water at pH 7.4 is 2.89.
IGALMI is for sublingual or buccal use. Each IGALMI sublingual film contains 120 mcg or 180 mcg of dexmedetomidine equivalent to 141.8 mcg and 212.7 mcg of dexmedetomidine hydrochloride, respectively.
IGALMI contains the following inactive ingredients: FD&C Blue #1 colorant, hydroxypropyl cellulose, peppermint oil, polyethylene oxide, and sucralose.
Igalmi side effects
Igalmi side effects are the unwanted reactions that may be experienced while using this medication
common side effects are
- Hypotension, Orthostatic Hypotension, and Bradycardia
- QT Interval Prolongation
- Somnolence
- Risk of Withdrawal Reactions
- Tolerance and Tachyphylaxis
Hypotension, Orthostatic Hypotension, and Bradycardia
IGALMI causes dose-dependent hypotension, orthostatic hypotension, and bradycardia. In clinical studies, 18%, 16%, and 9% of patients treated with 180 mcg of IGALMI, 120 mcg of IGALMI, and placebo, respectively, experienced orthostatic hypotension (defined as SBP decrease ≥ 20 mmHg or DBP decrease ≥ 10 mmHg after 1, 3, or 5 minutes of standing) at 2 hours post-dose. In those studies, 7%, 6%, and 1% of patients treated with 180 mcg of IGALMI, 120 mcg of IGALMI, and placebo, respectively, experienced HR ≤ 50 beats per minute within 2 hours of dosing, In clinical studies with IGALMI, patients were excluded if they had treatment with alpha-1 noradrenergic blockers, benzodiazepines, other hypnotics or antipsychotic drugs four hours prior to study drug administration; had a history of syncope or syncopal attacks; SBP < 110 mmHg; DBP < 70 mmHg; HR < 55 beats per minute; or had evidence of hypovolemia or orthostatic hypotension.
Reports of hypotension and bradycardia, including some resulting in fatalities, have been associated with the use of another dexmedetomidine product given intravenously (IGALMI is for sublingual or buccal use and is not approved for intravenous use). Clinically significant episodes of bradycardia and sinus arrest have been reported after administration of this other dexmedetomidine product to young, healthy adult volunteers with high vagal tone and when this product was given by rapid intravenous or bolus administration.
Because IGALMI decreases sympathetic nervous system activity, hypotension and/or bradycardia may be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension, and in geriatric patients.¶
Avoid use of IGALMI in patients with hypotension, orthostatic hypotension, advanced heart block, severe ventricular dysfunction, or history of syncope. After IGALMI administration, patients should be adequately hydrated and should sit or lie down until vital signs are within normal range. If a patient is unable to remain seated or lying down, precautions should be taken to reduce the risk of falls. Ensure that a patient is alert and not experiencing orthostatic hypotension or symptomatic hypotension prior to allowing them to resume ambulation
QT Interval Prolongation
IGALMI prolongs the QT interval. Avoid use of IGALMI in patients at risk of torsades de pointes or sudden death including those with known QT prolongation, a history of other arrhythmias, symptomatic bradycardia, hypokalemia, or hypomagnesemia, and in patients receiving other drugs known to prolong the QT interval.
Somnolence
IGALMI can cause somnolence. In placebo-controlled clinical studies in adults with agitation associated with schizophrenia or bipolar I or II disorder, somnolence (including fatigue and sluggishness) was reported in 23% and 22% of patients treated with IGALMI 180 mcg and 120 mcg, respectively, compared to 6% of placebo-treated patients. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or operating hazardous machinery, for at least eight hours after taking IGALMI.
Risk of Withdrawal Reactions
Symptoms of withdrawal have been observed after procedural sedation with another dexmedetomidine product administered intravenously. In this study, 12 (5%) adult patients who received intravenous dexmedetomidine up to 7 days (regardless of dose) experienced at least 1 event related to withdrawal within the first 24 hours after discontinuing dexmedetomidine and 7 (3%) adult patients who received intravenous dexmedetomidine experienced at least 1 event related with withdrawal 24 to 48 hours after discontinuing dexmedetomidine. The most common withdrawal reactions were nausea, vomiting, and agitation. In these subjects, tachycardia and hypertension requiring intervention occurred at a frequency of <5% in the 48 hours following intravenous dexmedetomidine discontinuation.
IGALMI was not studied for longer than 24 hours after the first dose. There may be a risk of physical dependence and a withdrawal syndrome if IGALMI is used in a manner other than indicated
Tolerance and Tachyphylaxis
Use of another dexmedetomidine product administered intravenously beyond 24 hours has been associated with tolerance and tachyphylaxis and a dose-related increase in adverse reactions.
IGALMI was not studied for longer than 24 hours after the first dose. There may be a risk of tolerance and tachyphylaxis if IGALMI is used in a manner other than indicated
Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
The safety of IGALMI was evaluated in 507 adult patients with agitation associated with schizophrenia (N=255) or bipolar I or II disorder (N=252) in two randomized, placebo-controlled studies (Studies 1 and 2) [see Clinical Studies (14)]. In both studies, patients were admitted to a clinical research unit or a hospital and remained under medical supervision for at least 24 hours following treatment. Patients were 18 to 71 years of age (mean age was 46 years old); 45% were female and 55% were male; 66% were Black, 31% were White, 2% were multiracial, and 1% were other.
In these studies, patients received an initial dose of IGALMI 180 mcg (N=252), IGALMI 120 mcg (N=255), or placebo (N=252). Patients who were hemodynamically stable (i.e., those with systolic blood pressure (SBP) > 90 mmHg, diastolic blood pressure (DBP) > 60 mmHg, and heart rate (HR) > 60 beats per minute) and without orthostatic hypotension (i.e., reduction in SBP < 20 mmHg or DBP < 10 mmHg upon standing) were eligible for an additional dose after 2 hours.
An additional half dose (90 mcg, 60 mcg, or placebo) was given to 7.1% (18/252), 22.7% (58/255) and 44.0% (111/252) of patients in the IGALMI 180 mcg, IGALMI 120 mcg or placebo arms, respectively. After at least an additional 2 hours, an additional second half dose (total IGALMI dose of 360 mcg, total IGALMI dose of 240 mcg, or placebo, respectively) was given to 3.2% (8/252), 9.4% (24/255), and 21.0% (53/252) of patients in the IGALMI 180 mcg, IGALMI 120 mcg or placebo arms, respectively.
In these studies, one patient discontinued treatment due to an adverse reaction of oropharyngeal pain. The most common adverse reactions (incidence ≥ 5% and at least twice the rate of placebo) were: somnolence, paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension.
Table 2 presents the adverse reactions that occurred in IGALMI-treated patients at a rate of at least 2% and at a higher rate than in placebo-treated patients in Studies 1 and 2.
Table 2: Adverse Reactions Reported in ≥2% of IGALMI-Treated Patients and Greater than Placebo in Two Placebo-Controlled Studies of Agitated Adult Patients with Schizophrenia or Bipolar I or II Disorder (Studies 1 and 2)
| Adverse Reaction | IGALMI 180 mcg
N=252 % |
IGALMI 120 mcg
N=255 % |
Placebo
N=252 % |
| Somnolence1 | 23 | 22 | 6 |
| Paresthesia or hypoesthesia oral | 7 | 6 | 1 |
| Dizziness | 6 | 4 | 1 |
| Hypotension | 5 | 5 | 0 |
| Orthostatic hypotension | 5 | 3 | <1 |
| Dry mouth | 4 | 7 | 1 |
| Nausea | 3 | 2 | 2 |
| Bradycardia | 2 | 2 | 0 |
| Abdominal discomfort2 | 2 | 0 | 1 |
1Somnolence includes the terms fatigue and sluggishness
2Abdominal discomfort includes dyspepsia, gastroesophageal reflux disease
Hypotension, Orthostatic Hypotension, and Bradycardia in Two Placebo-Controlled Studies In clinical studies, patients were excluded if they were treated with alpha-1 noradrenergic blockers, benzodiazepines, antipsychotic drugs, or other hypnotics four hours prior to study drug administration; had a history of syncope or syncopal attacks; their SBP was less than 110 mmHg; their DBP was less than 70 mmHg; their HR was less than 55 beats per minute; or they had evidence of hypovolemia or orthostatic hypotension. In these studies, vital signs were monitored (at 30 minutes, 1-, 2-, 4-, 6-, and 8hours post-dose), including orthostatic vital signs at 2-, 4-, and 8-hours post-dose. Maximum positional decreases in SBP and DBP after standing were observed at two hours post-dose. Maximal reductions on BP and HR were observed two hours post-dose.
Table 3 presents the mean BP and HR decrease across all patients from both studies at 2 hours post dose.
Table 3: Mean Blood Pressure and Heart Rate Decrease At 2 Hours Post-Dose
| IGALMI 180 mcg N=252 | IGALMI 120 mcg N=255 | Placebo N=252 | |
| Mean SBP Decrease (mmHg) | 15 | 13 | 1 |
| Mean DBP Decrease (mmHg) | 8 | 7 | <1 |
| Mean Heart Rate Decrease (bpm) | 9 | 7 | 3 |
In the clinical studies:
- 13%, 8%, and < 1% of patients in the single dose 180 mcg IGALMI, 120 mcg IGALMI, and placebo groups, respectively, experienced SBP ≤ 90 mmHg and a decrease ≥ 20 mmHg of SBP within 24 hours of dosing.
- 19%, 17%, and 2% of the patients in the 180 mcg IGALMI, 120 mcg IGALMI, and placebo groups, respectively, had a DBP ≤ 60 mmHg and a DBP decrease ≥ 10 mmHg within 24 hours of dosing.
- 4%, 3%, and 0% of patients in the 180 mcg IGALMI, 120 mcg IGALMI, and placebo groups, respectively, had a HR ≤ 50 beats per minute and a HR decrease ≥ 20 beats per minute within 24 hours of dosing.
At 8 hours post-dose, 2% of patients in the IGALMI 180 mcg group experienced a SBP ≤ 90 mmHg and decrease ≥ 20 mmHg compared with one patient (<1%) in the IGALMI 120 mcg group and none in the placebo group. At 24 hours, none of the patients in the IGALMI 180 mcg group experienced a SBP ≤90 mmHg and decrease ≥ 20 mmHg compared with one patient (<1%) in the IGALMI 120 mcg group and none in the placebo group. At 8 hours post-dose, none of the patients in the IGALMI 180 mcg group had a HR ≤ 50 beats per minute and a HR decrease ≥ 20 beats per minute compared with one patient in the 120 mcg group (<1%) and none in the placebo group.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of another
dexmedetomidine product given intravenously (IGALMI is not approved for intravenous use). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: Anemia
- Cardiac Disorders: Arrhythmia, atrial fibrillation, atrioventricular block, bradycardia, cardiac arrest, cardiac disorder, extrasystoles, myocardial infarction, supraventricular tachycardia, tachycardia, ventricular arrhythmia, ventricular tachycardia
- Eye Disorders: Photopsia, visual impairment
- Gastrointestinal Disorders: Abdominal pain, diarrhea, nausea, vomiting
- General Disorders and Administration Site Conditions: Chills, hyperpyrexia, pain, pyrexia, thirst
- Hepatobiliary Disorders: Hepatic function abnormal, hyperbilirubinemia
- Investigations: Alanine aminotransferase increased, aspartate aminotransferase increased, blood alkaline phosphatase increased, blood urea increased, electrocardiogram T wave inversion, gammaglutamyltransferase increased, electrocardiogram QT prolonged
- Metabolism and Nutrition Disorders: Acidosis, hyperkalemia, hypoglycemia, hypovolemia, hypernatremia
- Nervous System Disorders: Convulsion, dizziness, headache, neuralgia, neuritis, speech disorder
- Psychiatric Disorders: Agitation, confusional state, delirium, hallucination, illusion
- Renal and Urinary Disorders: Oliguria, polyuria
- Respiratory, Thoracic and Mediastinal Disorders: Apnea, bronchospasm, dyspnea, hypercapnia, hypoventilation, hypoxia, pulmonary congestion, respiratory acidosis
- Skin and Subcutaneous Tissue Disorders: Hyperhidrosis, pruritus, rash, urticaria
- Surgical and Medical Procedures: Light anesthesia
- Vascular Disorders: Blood pressure fluctuation, hemorrhage, hypertension, hypotension
