Xelstrym
Generic Name: dextroamphetamine
Dosage Form: transdermal system
Medically reviewed by A Ras, MD. Updated on April 5, 2022.
What is Xelstrym?
Xelstrym is an atypical central nerve system (CNS) stimulant prescription medication that is used to treat Attention Deficit Hyperactivity Disorder (ADHD) in adults and children aged 6 years old and over. It can help improve concentration and reduce impulsivity as well as hyperactivity in adults and children 6 years old or older who have ADHD.
It is not clear whether this medication can be used safely and effectively for children younger than 6 years old. age.
The Xelstrym drug is a federally controlled substance (CII) since it is a source of dextroamphetamine which is an ideal target for those who misuse prescription medications and street drugs. Make sure to keep Xelstrym in a secure place to guard it against theft. Don’t give it away to anyone else because it could cause harm or death. Giving away or selling Xelstrym could harm other people and is in violation of the law.
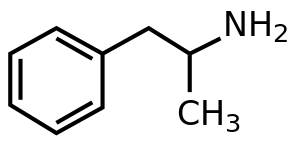
Xelstrym (dextroamphetamine) transdermal system, contains dextroamphetamine, a CNS stimulant. Dextroamphetamine is the dextro isomer of the compound d,l-amphetamine. The chemical name for dextroamphetamine is (2S)-1-phenylpropan-2-amine. It is a clear to slightly amber-colored liquid. The molecular weight of dextroamphetamine is 135.21 g/mol and the molecular formula is C9H13N.
Xelstrym is provided in four strengths: 4.5 mg/9 hours, 9 mg/9 hours, 13.5 mg/9 hours, and 18 mg/9 hours. The composition per unit area of all dosage strengths is identical. Inactive ingredients include acrylic adhesives, green ink, polyester/polyurethane backing, and polyester release liner.
Mechanism of Action
Amphetamines are non-catecholamine sympathomimetic amines with CNS stimulant activity. The exact mode of therapeutic action in ADHD is not known.
Important information
Xelstrym could have serious side effects which include:
- Dependence and abuse. Xelstrym and other amphetamine-containing products, as well as methylphenidate, have an increased risk of abuse and could cause physical or psychological dependence. Your healthcare provider should be able to check your child or you on the signs of abuse or dependence prior to or during your treatment.
- Inform your doctor If the child or you had any issues with addiction or abuse on prescription drugs, alcohol, or street drugs.
- Your doctor can inform your more details about the difference between dependence on physical and mental and addiction to drugs.
- Heart-related conditions, including:
- Stroke, sudden death, and heart attack in adult
- the sudden death of children with heart problems or heart defects
- higher cardiovascular rate, blood pressure
Your healthcare provider should be able to check you or your child for heart conditions prior to starting treatment. Discuss with your healthcare professional whether the child or you has any heart issues, heart problems, high blood pressure, or ancestry of any of these issues.
Your doctor should be checking your child’s or your own heart rate and blood pressure frequently throughout treatment.
Consult your physician or visit the nearest emergency room in a hospital immediately If the child or you shows any symptoms of heart trouble like chest discomfort, breathlessness, or fainting in treatment.
- Mental (psychiatric) problems, including:
- New or worse behavior, and mental health issues
- New or worse bipolar illness
- The symptoms of psychosis are often new (such as hearing voices or seeing or believing in things that aren’t actual) and new signs of manic/psychotic symptoms
Talk to your healthcare provider about any mental health issues you or your child may be suffering from or about a family history of bipolar disorder, suicide, or depression.
Contact your healthcare professional immediately if you or your child are experiencing any new or more severe mental issues or signs while receiving treatment, including hearing voices, believing or seeing things that aren’t real, or new manic symptoms.
Warnings and precautions
- Serious Cardiovascular Reactions: Sudden death has been reported in association with CNS stimulant treatment at recommended doses in pediatric patients with structural cardiac abnormalities or other serious heart problems. In adults, sudden death, stroke, and myocardial infarction have been reported. Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or coronary artery disease
- Blood Pressure and Heart Rate Increases: Monitor blood pressure and pulse. Consider benefits and risks before use in patients for whom blood pressure increases may be problematic
- Psychiatric Adverse Reactions: May cause psychotic or manic symptoms in patients with no prior history or exacerbation of symptoms in patients with pre-existing psychosis. Evaluate for bipolar disorder prior to Xelstrym use.
- Suppression of Growth: Monitor height and weight in pediatric patients during treatment
- Peripheral Vasculopathy, including Raynaud’s phenomenon: Stimulants are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Careful observation for digital changes is necessary during treatment with ADHD stimulants.
- Serotonin Syndrome: Increased risk when co-administered with serotonergic agents (e.g., SSRIs, SNRIs, triptans), but also during overdosage situations. If it occurs, discontinue Xelstrym and initiate supportive treatment.
- Contact Sensitization: Use of Xelstrym may lead to contact sensitization. Discontinue Xelstrym if contact sensitization is suspected.
- Application Site Reactions: During wear time or immediately after removal of Xelstrym, local skin reactions may occur. Select a different application site each day to limit the occurrence of skin reactions.
- External Heat: Avoid exposing Xelstrym to external heat sources during wear because both the rate and extent of absorption are increased. ¶
Who shouldn’t use Xelstrym?
Do not use Xelstrym in the event that either of you:
- people who are sensitive to amphetamine products or any other ingredients. Look below for a full listing of the ingredients.
- you have taken or are taking within the last 14 days taking a medication known as an inhibitor of monoamine oxidase (MAOI) that includes linezolid, an antibiotic, or the intravenous medicine Methylene Blue.
Before using Xelstrym
Before starting treatment, inform your healthcare professional about all health conditions, including the following if either you or your children
- suffer from heart issues such as heart defects, heart defects, or high blood pressure
- suffer from mental disorders, such as depression, or bipolar disorder, or have a family history of suicide, bipolar disorder, or depression
- are experiencing problems with circulation in their toes and fingers.
- Have kidney issues. Your physician may decrease the dose.
- Are pregnant or planning to get pregnant. It isn’t clear whether Xelstrym can affect the unborn baby.
- There is a registry for pregnant women for women who have been susceptible to Xelstrym during pregnancy. The aim of the registry is to gather data on the health of women exposed to Xelstrym as well as their infants. In the event that you are or your baby is pregnant while receiving treatment for Xelstrym discuss with your physician about registration for the National Pregnancy Registry for Psychiatric Medications by calling 1-866-961-2388 or going to the website at
https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/othermedications/.
- There is a registry for pregnant women for women who have been susceptible to Xelstrym during pregnancy. The aim of the registry is to gather data on the health of women exposed to Xelstrym as well as their infants. In the event that you are or your baby is pregnant while receiving treatment for Xelstrym discuss with your physician about registration for the National Pregnancy Registry for Psychiatric Medications by calling 1-866-961-2388 or going to the website at
- Are breastfeeding or planning to feed your baby. Xelstrym can be found in breast milk. Your child or you should not be breastfeeding during treatment. Discuss with your healthcare professional the best method to feed your child during treatment.
What other drugs can have an effect on Xelstrym?
Inform your doctor about every medication the child or you are taking for both prescription and over-the-counter medications as well as vitamins and herbal supplements.
It could alter the way other medicines function and other medicines could impact how Xelstrym performs. Utilizing Xelstrym in conjunction with other medications could result in serious side consequences. In some cases, the dosages of other medicines may need to be altered.
Tell your doctor whether either of you takes:
- selective serotonin Reuptake Inhibitors (SSRIs)
- medications that treat migraine headaches, called triptans
- lithium
- tramadol
- buspirone
- serotonin norepinephrine-reuptake inhibitors (SNRIs)
- tricyclic antidepressants
- Fentanyl
- Tryptophan
- St. John’s Wort
Your doctor will determine whether Xelstrym should be used in conjunction with other medications. Don’t begin any new treatment without consulting your healthcare professional first.
How can Xelstrym be utilized?
- Read your instructions for use included with your prescription for more details on the proper way to use, remove, and get rid of Xelstrym.
- Your doctor may alter the dosage as needed.
- Use one patch at any given time for no more than 9 hours. Apply only one patch every 24 hours. You must change the location of the application each time applying an update.
- Apply the patch two hours prior to when the effects are required and then remove it within 9 hours following application.
- Your doctor may suspend Xelstrym medication for a few days to look for ADHD symptoms.
When you suspect that your child or you consume excessively Xelstrym contact your physician or poison control at 1-800-222-1222 or visit the nearest emergency room in a hospital immediately.
Dosing information
The Usual Adult Dosage of ADHD
A recommended dose for starting for 9 mg per 9 hours. The maximum dose that is recommended is 18 mg/9 hour.
Use for treatment for Attention Deficit Hyperactivity Disorder (ADHD) for adults
Usual Dosage for Children with ADHD
A recommended dose for starting of 4.5 mg/9 hours. The dosage should be divided into weekly increments of 4.5 mg until a maximum dose that is 18 mg/9hrs
Use: Treatment for ADHD or Attention Deficit Disorder (ADHD) within infant patients (6 to 17 years old)
What should I be aware of when using Xelstrym?
- Don’t drive, operate machines that are heavy, or perform other dangerous tasks until you are aware of how Xelstrym impacts you.
- Following the application of your patch, you should avoid exposing the patch’s site to direct heat sources from outside like heat pads, hairdryers, electric blankets, and heat lamps, saunas hot tubs, hot water bed heaters. Exposure to heat could create too much medicine to be absorbed into your body and trigger dangerous adverse negative effects.
Xelstrym side effects
Xelstrym could have serious side effects which include:
- Read Important details.
- The slowing in growth (weight and size) for the children. Children should have their weight and height measured frequently throughout treatment. Your doctor may decide to stop treatment if your child isn’t growing in height or weight in the manner expected.
- Problems with circulation in fingers and toes (peripheral vasculopathy that includes Raynaud’s disease):
- Toes or fingers may be cold, numb, or painful
- Toes or fingers can alter the color from pale to blue, and finally red
Inform your doctor whether either you or your children suffer from an ache, numbness or color changes, or sensitivity to temperature changes in your toes or fingers.
Contact your healthcare professional immediately if your child shows any indications of wounds that are not explained appearing on your fingers or toes while receiving treatment.
- Serotonin Syndrome. A potentially life-threatening issue known as serotonin syndrome could develop when Xelstrym is combined with other medications. Lookup Before using Xelstrym, you must first know. Stop using Xelstrym and contact your physician or visit the nearest emergency room in the hospital immediately if you or your child exhibit any of the signs and indicators of serotonin syndrome:
- Affliction
- confusion
- fast heartbeat
- dizziness
- flushing
- muscle stiffness, muscles shaking
- seizures
- Hearing or seeing things that aren’t actual (hallucination)
- Coma
- variations in blood pressure
- sweating
- High blood temperature (hyperthermia)
- Lack of coordination
- nausea, vomiting, diarrhea
- Allergy to the skin (contact sensitization). Stop using Xelstrym immediately and notify your physician immediately in the event that the child or you experience blisters or swelling at or within the application site. It is possible that you are suffering from an allergy to the skin caused by Xelstrym. People with skin allergies to Xelstrym could develop an allergy to any amphetamine-containing products, including amphetamine supplements taken orally.
- Reactions to the application site have occurred in the past with Xelstrym during the time of wearing the patch, as well as following removal of the patch. The signs and indications of reactions to the application site include itching, pain redness, burning sensation, and swelling at the site of application. Consult your doctor when either you or your kid experiences any reactions to the application site that do not go away by themselves.
The most commonly reported adverse effects are:
- less appetite
- headache
- difficulty sleep
- Muscles that is twitching (tics)
- stomach pain
- vomiting
- nausea
- irritability
- higher blood pressure
- Heart rate increases
There are many possible adverse side negative effects. Consult your physician to get medical advice on adverse effects. You can report symptoms to FDA at 1-800-FDA-1088.
How do I keep Xelstrym in my storage?
- Storage Xelstrym at room temperature , between the 68degF-77degF range. (20degC between 25 and 25degC).
- Place Xelstrym in a secure place such as cabinets that are locked.
- Guard Xelstrym from moisture and light.
- Save Xelstrym in their sealed pouches up to the time you’re ready to utilize them.
- Remove any remaining unused or expired Xelstrym using an approved medication take-back program designated collection sites like pharmacies that sell retail, hospitals or clinic pharmacies, as well as law enforcement sites. If there is no take-back service or authorized collector is in place for each patch, it must remove it from its own pouch, removed from its liner that protects it and folded in half, and then disposed of in the same way as the patches that have been used.
Make sure that all medications are away from pets and children.
General information regarding the safe and efficient application of Xelstrym.
Some medicines are used for reasons other than those mentioned in the Medication Guide. Don’t use this medication for any medical condition for which it is not recommended. Don’t give it to others regardless of whether they are suffering from the same symptoms as you do. They could be harmed and is against the law. You may consult your doctor or pharmacist for advice that has been designed for healthcare professionals.
What are the ingredients of the Xelstrym?
Active ingredient: dextroamphetamine
Inactive ingredients: acrylic adhesives, green ink, polyester/polyurethane backing, and polyester release liner
Instructions For use
- (dextroamphetamine)
- transdermal system, CII
Xelstrym Instructions for Use contains information on how to apply, remove, and dispose of XELSTRYM transdermal system (patch).
XELSTRYM transdermal system (patch) is for use on skin only.
Read these Instructions for Use before you start using XELSTRYM and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child’s medical condition or treatment.
XELSTRYM is supplied in cartons containing 30 patches. Each patch is sealed in a pouch protecting it until you are ready to apply it.
Important information you need to know before applying XELSTRYM:
- Wear only 1 patch at a time
- A new patch must be applied every
- Do not wear XELSTRYM for more than 9 hours. Use only 1 patch per day (per 24 hours).
- When you apply a new patch, choose a different application site from the one used the previous day to reduce the chance of skin reactions such as irritation, discomfort or pain at the application site.
- Avoid touching the sticky side of XELSTRYM with your fingers. If you accidentally touch the sticky side of the patch, wash your hands right away with soap and water to remove any potential medicine that may have stuck to your fingers. Hand sanitizer should not be used in place of soap and water.
- Avoid exposing the application site to direct external heat sources, such as heating pads or electric blankets, heat lamps, saunas, hot tubs, heated water beds, and hair
- Check to see if the patch has become loose after bathing, showering or
- If the patch falls off:
- Do not reapply the same patch. Fold the patch in half so that the sticky sides are together and throw away the patch as instructed below in Steps 5 to 7.
- You should choose a different application site and apply a new
- The total wear time for the first patch that fell off and the second replacement patch should not total more than 9 hours in 1 day (24 hours).
- Parents or caregivers of children prescribed XELSTRYM should instruct the child to tell an adult if the patch becomes loose or falls
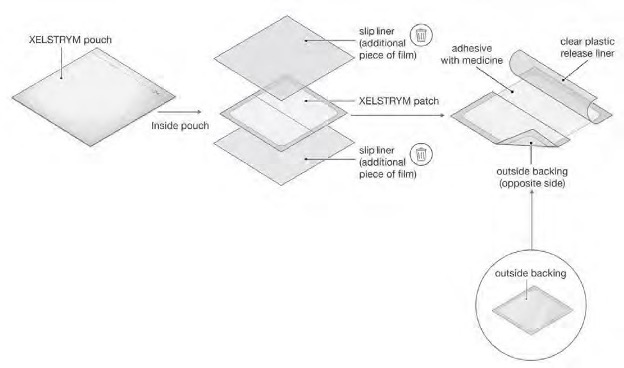
Figure A
Applying XELSTRYM
|
Step 1: Select the patch application site (see Figure B).
o chest o upper arm o upper back o flank (sides of the waist) o hip
|
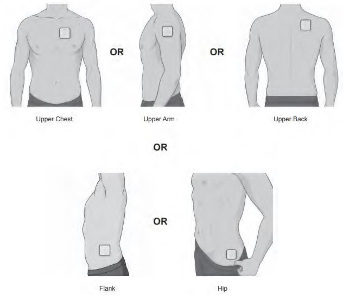
Figure B |
|
Step 2: Check the skin where XELSTRYM will be applied. Make sure it is: ● Not damaged (cut, scraped, burned), irritated (rashes), or has any other skin problems ● Dry (not wet) and clean, with no powder, oil, lotion, or gel |
|
| ● Hairless or nearly hairless
If there is hair at the patch application site, do not shave the application site. Use scissors to clip hair as close to the skin as possible. |
|
| Step 3: Applying XELSTRYM
● Carefully cut the pouch using scissors along the dashed line marked “Cut Here” (see Figure C).
● Gently remove the contents of the pouch and throw away the additional pieces of film above and below the patch (see Figure A, Figure D). ○ Do not use the patch if it is cut or damaged ○ Apply the patch right away after removing it from the pouch |
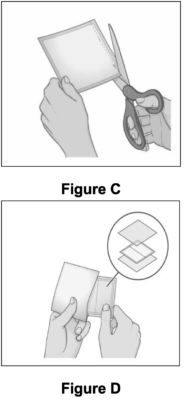 |
|
● Gently peel away half of the clear plastic liner. Avoid touching the sticky side with your fingers (see Figure E).
● Apply the sticky side of the patch to the application site you selected and smooth it down (see Figure F). |
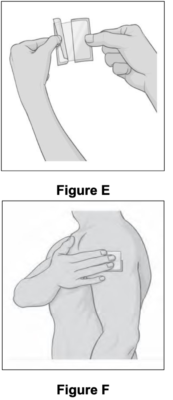 |
|
● Hold the edge of the remaining protective liner, slowly peel it off and smooth the patch down (see Figure G). ● Press the patch firmly with the palm of your hand. ● Smooth the patch to make sure it sticks well to your skin (See Figure H).
XELSTRYM patch should not be applied or reapplied with dressings, tape, or other adhesives. |
|
|
● Wash your hands with soap and water after applying the patch (see Figure I). Hand sanitizer should not be used in place of soap and water. |
|
|
Step 4: Check XELSTRYM regularly during the day to make sure it is completely sticking to the application site. Also check the patch when: ○ using the bathroom ○ undressing or changing clothes ○ after sleeping ○ if sweating a lot ○ after bathing, showering or swimming ● If patch edges lift, smooth the patch down on the skin and press firmly with the palm of your hand. XELSTRYM patch should not be reapplied with dressings, tape, or other adhesives. ● If the patch falls off, do not reapply the same patch. Fold the patch in half so that the sticky sides are together and throw away the patch as instructed below in Steps 5 to 7. You may apply a new patch to a different application site. The total wear time for the first patch that fell off and the second replacement patch should not be more than a total of 9 hours in 1 day (24 hours). ● Parents or caregivers of children prescribed XELSTRYM should instruct the child to tell an adult if the patch becomes loose or falls off. |
|
XELSTRYM Application Schedule for 9 Hour Wear Time:
| If you put the patch on at: | On the same day, remove the patch no later than: |
| 6:00 a.m. | 3:00 p.m. |
| 7:00 a.m. | 4:00 p.m. |
| 8:00 a.m. | 5:00 p.m. |
| 9:00 a.m. | 6:00 p.m. |
| 10:00 a.m. | 7:00 p.m. |
| 11:00 a.m. | 8:00 p.m. |
| 12:00 p.m. | 9:00 p.m. |
Removing and Disposing Used XELSTRYM:
|
Step 5: After 9 hours, remove XELSTRYM and fold it in half so that the sticky sides stick together (see Figure J) to avoid accidental exposure to medicine. ● If any adhesive (glue) remains on your skin after you remove the patch, apply an oil- based product or water and soap to the skin to remove the glue. |
Figure J |
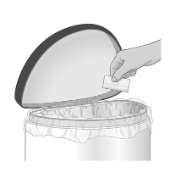
| Step 6: Throw away the used patch in a container with a lid right away (see Figure K). |
Figure K |
|
Step 7: Wash your hands with soap and water after throwing the patch away (see Figure L). Hand sanitizer should not be used in place of soap and water. |
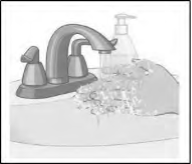
Figure L |
Storing XELSTRYM
- Store XELSTRYM at room temperature between 68º F to 77ºF (20ºC to 25ºC).
- Store XELSTRYM in a safe place, like a locked
- Protect XELSTRYM from light and
- Keep XELSTRYM in their unopened pouches until you are ready to use
- Keep XELSTRYM and all medicines out of the reach of
Disposing of unused XELSTRYM:
Dispose of remaining unused or expired XELSTRYM by a medication take-back program at authorized collection sites such as retail pharmacies, hospital or clinic pharmacies, and law enforcement locations. If no take-back program or authorized collector is available, each unused patch should be removed from its individual pouch, separated from the protective liner, folded in half, and disposed of in the same manner as used patches.
More details
Always consult your doctor to make sure the information presented on this page is applicable to your particular situation.

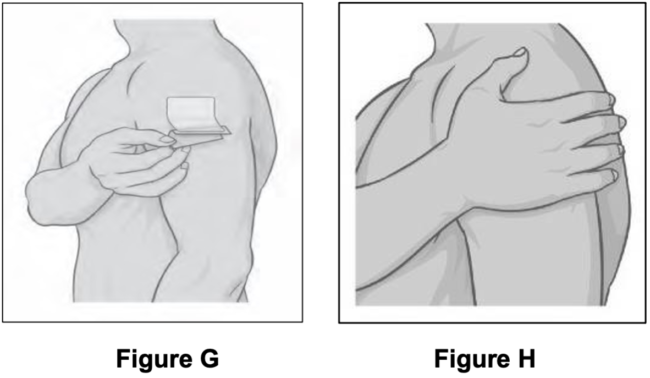
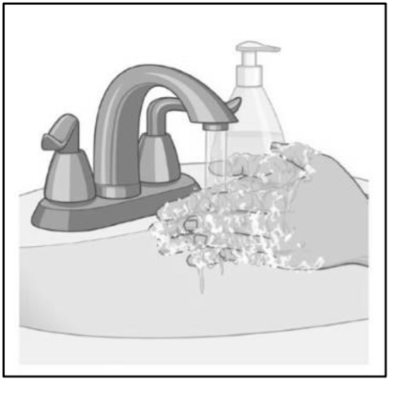 Figure I
Figure I