Zembrace SymTouch
Generic name: sumatriptan (injection)
Brand names: Imitrex, Imitrex Statdose, SUMAtriptan Succinate Syringe, Sumavel DosePro, Zembrace SymTouch
Drug class: Antimigraine agents
Medically reviewed by A Ras MD.
What is Zembrace SymTouch?
Zembrace SymTouch is a prescription medicine used to treat acute migraine headaches with or without aura in adults who have been diagnosed with migraine.
Zembrace SymTouch is not used to treat other types of headaches such as hemiplegic (that make you unable to move on one side of your body) or basilar (rare form of migraine with aura) migraines.
Zembrace SymTouch is not used to prevent or decrease the number of migraine you have. It is not known if Zembrace SymTouch is safe and effective in children under 18 years of age.
Description
ZEMBRACE SymTouch injection contains sumatriptan succinate, a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino) ethyl]-N- methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure:
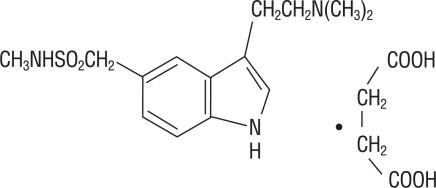
The empirical formula is C14H21N3O2S•C4H6O4, representing a molecular weight of 413.5. Sumatriptan succinate is a white to off-white powder that is readily soluble in water and in saline.
ZEMBRACE SymTouch is a clear, colorless to pale yellow, sterile, nonpyrogenic solution for subcutaneous injection. Each 0.5 mL of ZEMBRACE SymTouch contains 4.2 mg of sumatriptan succinate equivalent to 3-mg of sumatriptan (base) and 4.15 mg of sodium chloride, USP in Water for Injection, USP.
The pH range of solution is approximately 4.2 to 5.3 and the osmolality of injection is approximately 291 mOsmol (275 to 315 mOsmol).
Mechanism of Action
Sumatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine headache through agonist effects at the 5-HT1B/1D receptors on intracranial blood vessels and sensory nerves of the trigeminal system, which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release.
What is the most important information I should know about Zembrace SymTouch?
Zembrace SymTouch can cause serious side effects, including:
Heart attack and other heart problems. Heart problems may lead to death.
Stop taking Zembrace SymTouch and get emergency medical help right away if you have any of the following symptoms of a heart attack:
- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
Zembrace SymTouch is not for people with risk factors for heart disease unless a heart exam is done and shows no problem. You have a higher risk for heart disease if you:
- have high blood pressure
- have high cholesterol levels
- smoke
- are overweight
- have diabetes
- have a family history of heart disease
Who should not take Zembrace SymTouch?
Do not take Zembrace SymTouch if you have:
- heart problems or a history of heart problems
- narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease)
- uncontrolled high blood pressure
- hemiplegic migraines or basilar migraines. If you are not sure if you have these types of migraines, ask your healthcare provider.
- had a stroke, transient ischemic attacks (TIAs), or problems with your blood circulation
- severe liver problems
- an allergy to sumatriptan or any of the ingredients in Zembrace SymTouch. See the end of this guide for a complete list of ingredients in Zembrace SymTouch.
- taken any of the following medicines in the last 24 hours:
- almotriptan
- eletriptan
- frovatriptan
- naratriptan
- rizatriptan
- ergotamines
- dihydroergotamine
Ask your healthcare provider if you are not sure if your medicine is listed above.
What should I tell my healthcare provider before taking Zembrace SymTouch?
Before taking Zembrace SymTouch, tell your healthcare provider about all of your medical conditions, including if you:
- have high blood pressure
- have high cholesterol
- have diabetes
- smoke
- are overweight
- have heart problems or family history of heart problems or stroke
- have kidney problems
- have liver problems
- have had epilepsy or seizures
- are not using effective birth control
- are pregnant or plan to become pregnant. It is not known if Zembrace SymTouch can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Zembrace SymTouch passes into your breast milk. It is not known if this can harm your baby. Talk with your healthcare provider about the best way to feed your baby if you take Zembrace SymTouch.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Using Zembrace SymTouch with certain other medicines can affect each other, causing serious side effects.
Especially tell your healthcare provider if you take anti-depressant medicines called:
- selective serotonin reuptake inhibitors (SSRIs)
- serotonin norepinephrine reuptake inhibitors (SNRIs)
- tricyclic antidepressants (TCAs)
- monoamine oxidase inhibitors (MAOIs)
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take Zembrace Symtouch?
- Read the Instructions for use that come with Zembrace SymTouch.
- Certain people should take their first dose of Zembrace SymTouch in their healthcare provider’s office or in another medical setting. Ask your healthcare provider if you should take your first dose in a medical setting.
- Use Zembrace SymTouch exactly as your healthcare provider tells you to use it.
- Your healthcare provider may change your dose. Do not change your dose without first talking with your healthcare provider.
- For adults, the usual dose is a single injection given just below the skin.
- You should give an injection as soon as the symptoms of your headache start, but it may be given at any time during a migraine headache attack.
- If you did not get any relief after the first injection, do not give a second injection without first talking with your healthcare provider.
- You may use a second dose of Zembrace SymTouch after the first dose of Zembrace SymTouch OR after one dose of another sumatriptan medication separated by at least 1 hour, but not sooner, if your headache comes back or you only get some relief after your first injection.
- Do not take more than a total of 12 mg of Zembrace SymTouch in a 24-hour period. Talk to your doctor about how many Zembrace SymTouch you can take in a 24 hour period if you take another form of sumatriptan medication in between Zembrace SymTouch.
- If you use too much Zembrace SymTouch, call your healthcare provider or go to the nearest hospital emergency room right away.
- You should write down when you have headaches and when you take Zembrace SymTouch so you can talk with your healthcare provider about how Zembrace SymTouch is working for you.
What should I avoid while taking Zembrace Symtouch?
Zembrace SymTouch can cause dizziness, weakness, or drowsiness. If you have these symptoms, do not drive a car, use machinery, or do anything where you need to be alert.
What are the possible side effects of Zembrace SymTouch?
See “What is the most important information I should know about Zembrace SymTouch?” Zembrace SymTouch may cause serious side effects, including:
- changes in color or sensation in your fingers and toes (Raynaud’s syndrome)
- stomach and intestinal problems (gastrointestinal and colonic ischemic events). Symptoms of gastrointestinal and colonic ischemic events include:
- sudden or severe stomach pain
- stomach pain after meals
- weight loss
- nausea or vomiting
- constipation or diarrhea
- bloody diarrhea
- fever
- problems with blood circulation to your legs and feet (peripheral vascular ischemia).
Symptoms of peripheral vascular ischemia include:- cramping and pain in your legs or hips
- feeling of heaviness or tightness in your leg muscles
- burning or aching pain in your feet or toes while resting
- numbness, tingling, or weakness in your legs
- cold feeling or color changes in 1 or both legs or feet
- hives (itchy bumps); swelling of your tongue, mouth, or throat.
- medication overuse headaches. Some people who use too many Zembrace SymTouch injections may have worse headaches (medication overuse headache). If your headaches get worse, your healthcare provider may decide to stop your treatment with Zembrace SymTouch.
- serotonin syndrome. Serotonin syndrome is a rare but serious problem that can happen in people using Zembrace SymTouch, especially if Zembrace SymTouch is used with anti-depressant medicines called SSRIs or SNRIs.Call your healthcare provider right away if you have any of the following symptoms of serotonin syndrome:
- mental changes such as seeing things that are not there (hallucinations), agitation, or coma
- fast heartbeat
- changes in blood pressure
- high body temperature
- tight muscles
- trouble walking
- seizures. Seizures have happened in people taking Zembrace SymTouch who have never had seizures before. Talk with your healthcare provider about your chance of having seizures while you take Zembrace SymTouch.
The most common side effects of Zembrace SymTouch include:
- pain or redness at your injection site
- tingling or numbness in your fingers or toes
- dizziness
- warm, hot, burning feeling to your face (flushing)
- discomfort or stiffness in your neck
- feeling weak, drowsy, or tired
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Zembrace SymTouch. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1- 800-FDA-1088.
General information about the safe and effective use of Zembrace SymTouch
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use Zembrace SymTouch for a condition for which it was not prescribed. Do not give Zembrace SymTouch to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Zembrace SymTouch. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Zembrace SymTouch that is written for healthcare professionals.
For more information, go to www.drreddys.com or call 1-888-966-8766.
How should I store Zembrace SymTouch?
- Store between 68° and 77°F (20° and 25°C)
- Store your medicine away from light.
- Keep your medicine in the packaging or carrying case provided with it.
Keep Zembrace SymTouch and all medicines out of the reach of children.
What are the ingredients in Zembrace SymTouch?
Active ingredient: sumatriptan succinate
Inactive ingredients: sodium chloride, water for injection
Instructions for use for Zembrace SymTouch
Zembrace SymTouch (Zem-brace Sim-Touch)
(sumatriptan succinate)
Injection
The Zembrace SymTouch Autoinjector is a single-use, pre-filled injection device with the exact dose you need.
The device should be used only 1 time and then discarded.
 Important Precautions
Important Precautions
- Keep out of reach of children.
- Do not use after the expiration date has passed.
- Do not store or expose to high temperatures.
- Do not freeze.
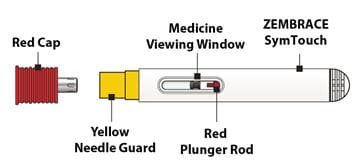
- Do not remove the Red Cap until you are ready to inject.
- Do not use if dropped after removing the Red Cap.
- Do not put or press thumb, fingers or hand over the Yellow Needle Guard.
Step 1 – Inspect Autoinjector and Gather Supplies
1A- Remove Autoinjector from the carton.
1B – Check the Expiration Date on the Autoinjector
Do not use if the expiration date has passed.
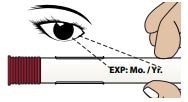
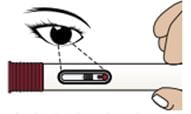
1C – Inspect the medicine through the Medicine Viewing Window
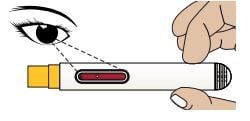
Inspect the appearance of Zembrace SymTouch Autoinjector medicine through the medicine window. It must be a clear, colorless to pale yellow solution.
Do not inject the medicine if the solution looks discolored or cloudy or contains lumps, flakes, or particles.
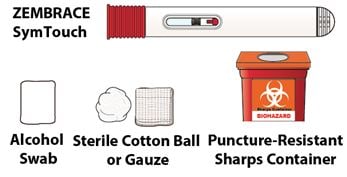
1D – Gather the following supplies
- Autoinjector
- Alcohol Swab
- Sterile Cotton Ball or Gauze
- Puncture-Resistant Sharps Container
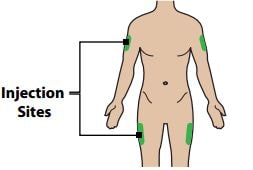
Step 2 – Choose and Prepare Your Injection Site
2A – Choose your injection site
The recommended injection sites are the sides of the thighs and the arms.
Caution: Do not inject where your skin is scarred, bruised, tender, red or near any open wound.
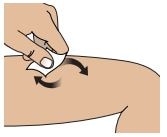
2B – Clean the injection site with an alcohol swab
Do not touch injection site area after cleaning.
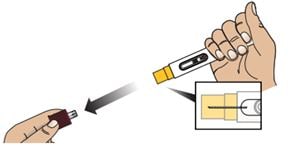
2C – Remove the Red Cap
Hold the body of the Autoinjector in one hand and pull the Red Cap straight off.
Warning: Do not put or press thumb, fingers or hand over the Yellow Needle Guard, doing so may result in a needle stick injury.
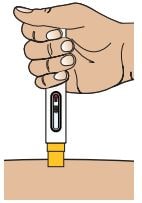
Step 3 – Inject the Medicine
3A – Place Autoinjector straight on your injection site
Place the Autoinjector straight on your injection site (90 degrees) with the Yellow Needle Guard end gently pressed against your skin.
3B – Perform Injection
Press and hold the Autoinjector down against your skin. You will hear the first “Click 1” as the injection starts. Continue to hold the device down until you hear the second “Click 2.”
Wait 5 seconds before you remove the Autoinjector!
After “Click 2,” continue to hold down and slowly count to 5 to make sure you receive your full dose.
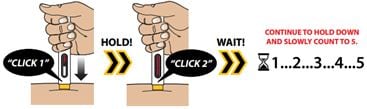
Important: To receive your full dose, always hold the Autoinjector down on your injection site for additional time after you hear the second click. This allows all of the medicine to be delivered.
3C – Remove Autoinjector from skin
Remove the Autoinjector by lifting it straight away from your skin. The Yellow Needle Guard will drop down and lock over the needle.
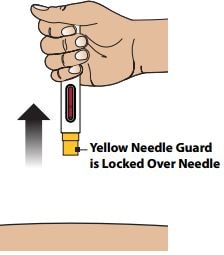
Step 4 – Confirm Injection and Dispose the Autoinjector
4A – Confirm Red Color in Medicine Viewing Window
Look to confirm that the Red Plunger Rod has filled the Medicine Viewing Window. This means you received the full dose.
Caution: Call your healthcare provider if the Red Plunger Rod has not filled the Medicine Viewing Window. This may mean that only a partial dose was delivered.
Do not try to reuse the Autoinjector.
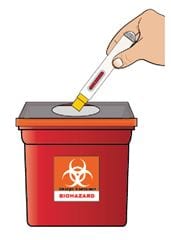
4B Disposal of the Autoinjector
Put your used Autoinjector, caps, needles, and sharps in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash. Do not recycle your used sharps disposal container.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is: made of a heavy-duty plastic; can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out; upright and stable during use; leak-resistant; and properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
If needed, make sure you get a refill of your Autoinjector.
4C – Treat Injection Site
Treat the injection site with a cotton ball, gauze pad or bandage, as needed.
Do not rub the injection site.
Label
PRINCIPAL DISPLAY PANEL – NDC: 67857-809-37 – AUTOINJECTOR LABEL

PRINCIPAL DISPLAY PANEL – NDC: 67857-809-38 – 4-COUNT CARTON LABEL (PARTITION)