Imitrex
Generic name: sumatriptan (injection)
Brand names: Imitrex, Imitrex Statdose, SUMAtriptan Succinate Syringe, Sumavel DosePro, Zembrace SymTouch
Drug class: Antimigraine agents
What is Imitrex?
Imitrex is a prescription medicine used to treat acute migraine headaches with or without aura in adults.
Imitrex is not used to treat other types of headaches such as hemiplegic (that make you unable to move on one side of your body) or basilar (rare form of migraine with aura) migraines.
Imitrex is not used to prevent or decrease the number of migraine headaches you have.
It is not known if Imitrex is safe and effective to treat cluster headaches.
It is not known if Imitrex is safe and effective in children under 18 years of age.
Description
IMITREX injection contains sumatriptan succinate, a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure:
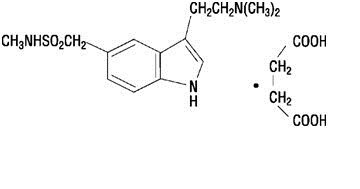
The empirical formula is C14H21N3O2S•C4H6O4, representing a molecular weight of 413.5. Sumatriptan succinate is a white to off-white powder that is readily soluble in water and in saline.
IMITREX injection is a clear, colorless to pale yellow, sterile, nonpyrogenic solution for subcutaneous injection. Each 0.5 mL of IMITREX injection 8-mg/mL solution contains 5.6 mg of sumatriptan succinate equivalent to 4 mg of sumatriptan and 3.8 mg of sodium chloride, USP in Water for Injection, USP. Each 0.5 mL of IMITREX injection 12-mg/mL solution contains 8.4 mg of sumatriptan succinate equivalent to 6 mg of sumatriptan and 3.5 mg of sodium chloride, USP in Water for Injection, USP. The pH range of both solutions is approximately 4.2 to 5.3. The osmolality of both injections is 291 mOsmol.
Mechanism of Action
Sumatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine and cluster headaches through agonist effects at the 5-HT1B/1D receptors on intracranial blood vessels and sensory nerves of the trigeminal system, which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release.
What is the most important information I should know about Imitrex?
Imitrex can cause serious side effects, including:
Heart attack and other heart problems. Heart problems may lead to death.
Stop taking Imitrex and get emergency medical help right away if you have any of the following symptoms of a heart attack:
- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
Imitrex is not for people with risk factors for heart disease unless a heart exam is done and shows no problem. You have a higher risk for heart disease if you:
- have high blood pressure
- have high cholesterol levels
- smoke
- are overweight
- have diabetes
- have a family history of heart disease
Who should not take Imitrex?
Do not take Imitrex if you have:
- an allergy to sumatriptan or any of the ingredients in Imitrex. See the end of this leaflet for a complete list of ingredients in Imitrex.
- heart problems or a history of heart problems
- narrowing of blood vessels to your legs, arms, stomach, or kidneys (peripheral vascular disease)
- uncontrolled high blood pressure
- severe liver problems
- hemiplegic migraines or basilar migraines. If you are not sure if you have these types of migraines, ask your healthcare provider.
- had a stroke, transient ischemic attacks (TIAs), or problems with your blood circulation
- taken any of the following medicines in the last 24 hours:
- almotriptan (Axert)
- frovatriptan (Frova)
- rizatriptan (Maxalt, Maxalt-Mlt)
- ergotamines (Cafergot, Ergomar, Migergot)
- eletriptan (Relpax)
- naratriptan (Amerge)
- sumatriptan and naproxen (Treximet)
- dihydroergotamine (D.H.E. 45, Migranal)
Ask your healthcare provider if you are not sure if your medicine is listed above.
What should I tell my healthcare provider before taking Imitrex?
Before you take Imitrex, tell your healthcare provider about all of your medical conditions,
Including if you:
- have high blood pressure.
- have high cholesterol.
- have diabetes.
- smoke.
- are overweight.
- have heart problems or family history of heart problems or stroke.
- have kidney problems.
- have liver problems.
- have had epilepsy or seizures.
- are not using effective birth control.
- are pregnant or plan to become pregnant. It is not known if Imitrex can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Imitrex passes into your breast milk. It is not known if this can harm your baby. Talk with your healthcare provider about the best way to feed your baby if you take Imitrex.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Imitrex and certain other medicines can affect each other, causing serious side effects.
Especially tell your healthcare provider if you take antidepressant medicines called:
- selective serotonin reuptake inhibitors (SSRIs)
- serotonin norepinephrine reuptake inhibitors (SNRIs)
- tricyclic antidepressants (TCAs)
- monoamine oxidase inhibitors (MAOIs)
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take Imitrex?
- Certain people should take their first dose of Imitrex in their healthcare provider’s office or in another medical setting. Ask your healthcare provider if you should take your first dose in a medical setting.
- Take Imitrex exactly as your healthcare provider tells you to take it.
- Your healthcare provider may change your dose. Do not change your dose without first talking to your healthcare provider.
- Take Imitrex tablets whole with water or other liquids.
- If you do not get any relief after your first tablet, do not take a second tablet without first talking with your healthcare provider.If your headache comes back or you only get some relief from your headache, you can take a second tablet 2 hours after the first tablet.
- Do not take more than 200 mg of Imitrex tablets in a 24‑hour period.
- If you take too much Imitrex, call your healthcare provider or go to the nearest hospital emergency room right away.
- You should write down when you have headaches and when you take Imitrex so you can talk with your healthcare provider about how Imitrex is working for you.
What should I avoid while taking Imitrex?
Imitrex can cause dizziness, weakness, or drowsiness. If you have these symptoms, do not drive a car, use machinery, or do anything where you need to be alert.
What are the possible side effects of Imitrex?
Imitrex may cause serious side effects. See “What is the most important information I should know about Imitrex?”
These serious side effects include:
- changes in color or sensation in your fingers and toes (Raynaud’s syndrome)
- stomach and intestinal problems (gastrointestinal and colonic ischemic events). Symptoms of gastrointestinal and colonic ischemic events include:
- sudden or severe stomach pain
- stomach pain after meals
- weight loss
- fever
- nausea or vomiting
- constipation or diarrhea
- bloody diarrhea
- Problems with blood circulation to your legs and feet (peripheral vascular ischemia). Symptoms of peripheral vascular ischemia include:
- cramping and pain in your legs or hips
- feeling of heaviness or tightness in your leg muscles
- burning or aching pain in your feet or toes while resting
- numbness, tingling, or weakness in your legs
- cold feeling or color changes in 1 or both legs or feet
- Medication overuse headaches. Some people who use too many Imitrex tablets may have worse headaches (medication overuse headache). If your headaches get worse, your healthcare provider may decide to stop your treatment with Imitrex.
- Serotonin syndrome. Serotonin syndrome is a rare but serious problem that can happen in people using Imitrex, especially if Imitrex is used with anti-depressant medicines called SSRIs or SNRIs. Call your healthcare provider right away if you have any of the following symptoms of serotonin syndrome:
- mental changes such as seeing things that are not there (hallucinations), agitation, or coma
- fast heartbeat
- changes in blood pressure
- high body temperature
- tight muscles
- trouble walking
- Hives (itchy bumps); swelling of your tongue, mouth, or throat.
- Seizures. Seizures have happened in people taking Imitrex who have never had seizures before. Talk with your healthcare provider about your chance of having seizures while you take Imitrex.
The most common side effects of Imitrex tablets include:
- tingling or numbness in your fingers or toes
- warm or cold feeling
- feeling weak, drowsy, or tired
- pain, discomfort, or stiffness in your neck, throat, jaw, or chest
- dizziness
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Imitrex. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Imitrex.
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use Imitrex for a condition for which it was not prescribed. Do not give Imitrex to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Imitrex. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Imitrex that is written for healthcare professionals.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0173-0739-02
- IMITREX
- (SUMATRIPTAN)
- INJECTION
- 4 mg
- 0.5 mL
- For subcutaneous injection only.
- 2 Single-Dose Syringe Cartridges
- Rx only
- 62000000052698 Rev. 8/20
PRINCIPAL DISPLAY PANEL
- NDC 0173-0478-00
- IMITREX
- (SUMATRIPTAN)
- INJECTION
- 6 mg
- 0.5 mL
- For subcutaneous injection only.
- Rx only
- 2 Single-Dose Syringe Cartridges
- 62000000052700 Rev. 8/20
PRINCIPAL DISPLAY PANEL
- NDC 0173-0479-00
- IMITREX
- STATdose SYSTEM
- 6 mg
- This carton contains:

SRC: NLM .


