Uloric
Generic name: febuxostat
Drug class: Antihyperuricemic agents
Medically reviewed by A Ras MD.
What is Uloric?
Uloric is a prescription medicine called a xanthine oxidase (XO) inhibitor used to lower blood uric acid levels in adult patients with gout when allopurinol has not worked well enough or when allopurinol is not right for you.
Uloric is not for use in people who do not have symptoms of high blood uric acid levels.
It is not known if Uloric is safe and effective in children.
Description
ULORIC (febuxostat) is a xanthine oxidase inhibitor. The active ingredient in ULORIC is 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid, with a molecular weight of 316.38. The empirical formula is C16H16N2O3S.
The chemical structure is:
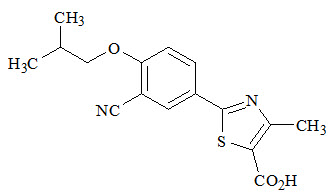
Febuxostat is a non-hygroscopic, white crystalline powder that is freely soluble in dimethylformamide; soluble in dimethylsulfoxide; sparingly soluble in ethanol; slightly soluble in methanol and acetonitrile; and practically insoluble in water. The melting range is 205°C to 208°C.
ULORIC tablets for oral use contain the active ingredient, febuxostat, and are available in two dosage strengths, 40 mg and 80 mg. Inactive ingredients include lactose monohydrate, microcrystalline cellulose, hydroxypropyl cellulose, sodium croscarmellose, silicon dioxide and magnesium stearate. ULORIC tablets are coated with Opadry II, green.
Mechanism of Action
ULORIC, a xanthine oxidase inhibitor, achieves its therapeutic effect by decreasing serum uric acid. ULORIC is not expected to inhibit other enzymes involved in purine and pyrimidine synthesis and metabolism at therapeutic concentrations.
What is the most important information that I should know about Uloric?
Uloric may cause serious side effects, including:
- Heart -related deaths.
Call your doctor or get emergency medical help right away if you have any of the following symptoms, especially if they are new, worse, or worry you:
- chest pain
- shortness of breath or trouble breathing
- dizziness, fainting or feeling lightheaded
- rapid or irregular heartbeat
- numbness or weakness on one side of your body
- slurring of speech
- sudden blurry vision or sudden severe headache
Who should not take Uloric?
Do not take Uloric if you:
- take azathioprine (Azasan, Imuran)
- take mercaptopurine (Purinethol, Purixan)
What should I tell my healthcare provider before taking Uloric?
Before taking Uloric tell your doctor about all of your medical conditions, including if you:
- have taken allopurinol and what happened to you while you were taking it.
- have a history of heart disease or stroke.
- have liver or kidney problems.
- are pregnant or plan to become pregnant. It is not known if Uloric will harm your unborn baby. Talk with your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if Uloric passes into your breast milk. You and your doctor should decide if you should take Uloric while breastfeeding.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Uloric may affect the way other medicines work, and other medicines may affect how Uloric works.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take Uloric?
- Take Uloric exactly as your doctor tells you to take it.
- Uloric can be taken with or without food.
- Uloric can be taken with antacids.
- Your gout may get worse (flare) when you start taking Uloric. Do not stop taking Uloric because you have a flare.
Your doctor may do certain tests while you take Uloric.
What are the possible side effects of Uloric?
Uloric may cause serious side effects, including:
- Heart problems. See “What is the most important information I should know about Uloric?”
- Gout Flares. Gout flares can happen when you start taking Uloric. Your doctor may give you other medicines to help prevent your gout flares.
- Liver problems. Liver problems can happen in people who take Uloric. Your doctor may do blood tests to check how well your liver is working before and during your treatment with Uloric. Tell your doctor if you get any of the following signs or symptoms of liver problems:
- fatigue
- loss of appetite for several days or longer
- pain, aching, or tenderness on the right side of your stomach-area
- dark or “tea-colored” urine
- your skin or the white part of your eyes turns yellow (jaundice)
- Severe skin and allergic reactions. Serious skin and allergic reactions that may affect different parts of the body such as your liver, kidneys, heart or lungs, can happen in people who take Uloric. Call your doctor right away or get emergency medical help if you have any of the following symptoms:
- rash
- red and painful skin
- severe skin blisters
- peeling skin
- sores around the lips, eyes or mouth
- swollen face, lips, mouth, tongue or throat
- flu-like symptoms
The most common side effects of Uloric include:
- abnormal liver function tests
- nausea
- joint pain
- rash
These are not all of the possible side effects of Uloric.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Uloric.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Uloric for a condition for which it was not prescribed. Do not give Uloric to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your doctor or pharmacist for information about Uloric that is written for health professionals.
How should I store Uloric?
- Store Uloric at room temperature.
- Keep Uloric out of the light.
Keep Uloric and all medicines out of the reach of children.
What are the ingredients in Uloric?
Active ingredient: febuxostat
Inactive ingredients: lactose monohydrate, microcrystalline cellulose, hydroxypropyl cellulose, sodium croscarmellose, silicon dioxide, magnesium stearate, and Opadry II, green
For more information, go to www.ULORIC.com or call 1-877-TAKEDA (1-877-825-3327).
Label
PRINCIPAL DISPLAY PANEL – 40 MG TABLET BOTTLE LABEL
- NDC 64764-918-30
30 Tablets - Uloric
(febuxostat)
tablets - 40 mg
- Dispense the accompanying
Medication Guide to each patient.

PRINCIPAL DISPLAY PANEL – 80 MG TABLET BOTTLE LABEL
- NDC 64764-677-30
30 Tablets - Uloric
(febuxostat)
tablets - 80 mg
- Dispense the accompanying
Medication Guide to each patient.


