Thalomid
Generic name: thalidomide
Drug classes: Leprostatics, Miscellaneous antineoplastics, Other immunosuppressants
Medically reviewed by A Ras MD.
What is Thalomid?
Thalomid is a prescription medicine used to treat people who have been newly diagnosed with multiple myeloma (MM) in combination with the medicine dexamethasone. It is also used to treat people who have moderate to severe new lesions of leprosy. Thalomid is not used by itself to treat the skin lesions when there is moderate to severe nerve pain. It is used as a maintenance treatment to prevent and keep the skin lesions of leprosy from coming back (recurring).
It is not known if Thalomid is safe and effective in children under 12 years of age.
Description
THALOMID, α-(N-phthalimido) glutarimide, is an immunomodulatory agent. The empirical formula for thalidomide is C13H10N2O4 and the gram molecular weight is 258.2. The CAS number of thalidomide is 50-35-1.
| Chemical Structure of Thalidomide | |
 |
|
| Note: ∙ = asymmetric carbon at | |
Thalidomide is an off-white to white, odorless, crystalline powder that is soluble at 25°C in dimethyl sulfoxide and sparingly soluble in water and ethanol. The glutarimide moiety contains a single asymmetric center and, therefore, may exist in either of two optically active forms designated S-(-) or R-(+). THALOMID is an equal mixture of the S-(-) and R-(+) forms and, therefore, has a net optical rotation of zero.
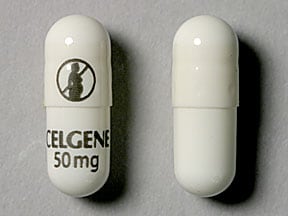
THALOMID is available in 50 mg, 100 mg, 150 mg and 200 mg capsules for oral administration. Active ingredient: thalidomide. Inactive ingredients: pregelatinized starch and magnesium stearate. The 50 mg capsule shell contains gelatin, titanium dioxide, and black ink. The 100 mg capsule shell contains black iron oxide, yellow iron oxide, titanium dioxide, gelatin, and black ink. The 150 mg capsule shell contains FD&C blue #2, black iron oxide, yellow iron oxide, titanium dioxide, gelatin, and black and white ink. The 200 mg capsule shell contains FD&C blue #2, titanium dioxide, gelatin, and white ink.
Mechanism of Action
The mechanism of action of THALOMID is not fully understood. Cellular activities of thalidomide are mediated through its target cereblon, a component of a cullin ring E3 ubiquitin ligase enzyme complex. THALOMID possesses immunomodulatory, anti-inflammatory and antiangiogenic properties. Available data from in vitro studies and clinical trials suggest that the immunologic effects of this compound can vary substantially under different conditions, but may be related to suppression of excessive tumor necrosis factor-alpha (TNF-α) production and down-modulation of selected cell surface adhesion molecules involved in leukocyte migration.
For example, administration of thalidomide has been reported to decrease circulating levels of TNF-α in patients with erythema nodosum leprosum (ENL); however, it has also been shown to increase plasma TNF-α levels in HIV-seropositive patients. Other anti-inflammatory and immunomodulatory properties of thalidomide may include suppression of macrophage involvement in prostaglandin synthesis, and modulation of interleukin-10 and interleukin-12 production by peripheral blood mononuclear cells. Thalidomide treatment of multiple myeloma patients is accompanied by an increase in the number of circulating natural killer cells, and an increase in plasma levels of interleukin-2 and interferon-gamma (T cell-derived cytokines associated with cytotoxic activity). Thalidomide was found to inhibit angiogenesis in a human umbilical artery explant model in vitro. The cellular processes of angiogenesis inhibited by thalidomide may include the proliferation of endothelial cells.
What is the most important information I should know about Thalomid?
Before you begin taking Thalomid, you must read and agree to all of the instructions in the Thalomid REMS program. Before prescribing Thalomid, your healthcare provider will explain the Thalomid REMS program to you and have you sign the Patient-Physician Agreement Form.
Thalomid can cause serious side effects including:
- Severe and life-threatening human birth defects (deformed babies) or death of an unborn baby. Females who are pregnant or who plan to become pregnant must not take Thalomid.
- Females must not get pregnant:
- For at least 4 weeks before starting Thalomid
- While taking Thalomid
- During any breaks (interruptions) in your treatment with Thalomid
- For at least 4 weeks after stopping Thalomid
- Females who can become pregnant:
- Will have pregnancy tests weekly for 4 weeks, then every 4 weeks if your menstrual cycle is regular, or every 2 weeks if your menstrual cycle is irregular.
- If you miss your period or have unusual bleeding, you will need to have a pregnancy test and receive counseling.
- Must agree to use two acceptable forms of birth control at the same time, for at least 4 weeks before, while taking, during any breaks (interruptions) in your treatment, and for at least 4 weeks after stopping Thalomid.
- Talk with your healthcare provider to find out about options for acceptable forms of birth control that you may use to prevent pregnancy before, during, and after treatment with Thalomid.
- Stop taking Thalomid and call your healthcare provider right away if you have unprotected sex or if you think your birth control has failed.
- If you become pregnant while taking Thalomid, stop taking it right away and call your healthcare provider. If your healthcare provider is not available, you should call Celgene Customer Care Center at 1-888-423-5436. Healthcare providers and patients should report all cases of pregnancy to:
- FDA MedWatch at 1-800-FDA-1088, and
- Celgene Corporation at 1-888-423-5436
- There is a pregnancy exposure registry that monitors the outcomes of females who take Thalomid during pregnancy, or if their male partner takes Thalomid and they are exposed during pregnancy. You can enroll in this registry by calling Celgene Corporation at the phone number listed above.
- Thalomid can pass into human semen:
- Males, including those who have had a vasectomy, must always use a latex or synthetic condom during any sexual contact with a pregnant female or a female that can become pregnant while taking Thalomid, during any breaks (interruptions) in your treatment with Thalomid, and for up to 4 weeks after stopping Thalomid.
- Do not have unprotected sexual contact with a female who is or could become pregnant. Tell your healthcare provider if you do have unprotected sexual contact with a female who is or could become pregnant.
- Do not donate sperm while taking Thalomid, during any breaks (interruptions) in your treatment, and for 4 weeks after stopping Thalomid. If a female becomes pregnant with your sperm, the baby may be exposed to Thalomid and may be born with birth defects.Men, if your female partner becomes pregnant, you should call your healthcare provider right away.
- Females must not get pregnant:
- Blood clots. People with multiple myeloma (MM) who take Thalomid may have an increased risk for blood clots in their arteries, veins, and lungs. This risk is even higher if you take the medicine dexamethasone with Thalomid to treat your MM. Heart attacks and strokes may also happen if you take Thalomid with dexamethasone.
Before taking Thalomid, tell your healthcare provider about all the medicines you take. Certain other medicines can also increase your risk for blood clots.
Call your healthcare provider or get medical help right away if you get any of the following during treatment with Thalomid:- Signs or symptoms of a blood clot in the lung, arm, or leg may include: shortness of breath, chest pain, or arm or leg swelling
- Signs or symptoms of a heart attack may include: chest pain that may spread to the arms, neck, jaw, back, or stomach area (abdomen), feeling sweaty, shortness of breath, feeling sick or vomiting
- Signs or symptoms of stroke may include: sudden numbness or weakness, especially on one side of the body, severe headache or confusion, or problems with vision, speech, or balance
Who should not take Thalomid?
Do not take Thalomid if you:
- are pregnant, plan to become pregnant, or become pregnant during treatment with Thalomid. See “What is the most important information I should know about Thalomid?”
- are allergic to thalidomide or any of the ingredients in Thalomid. See the end of this Medication Guide for a complete list of ingredients in Thalomid.
What should I tell my healthcare provider before taking Thalomid?
Before you take Thalomid, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of seizures
- drink alcohol
- plan to have surgery
- are breastfeeding. Thalomid must not be used by females who are breastfeeding. It is not known if Thalomid passes into your breast milk and can harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Thalomid and other medicines may affect each other, causing serious side effects. Talk with your healthcare provider before taking any new medicines. Certain medicines can affect the way that birth control pills, injections, transdermal systems (patches), or implants work. You could become pregnant. See “What is the most important information I should know about Thalomid?”
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist.
How should I take Thalomid?
- Take Thalomid exactly as prescribed and follow all the instructions of the Thalomid REMS program.
- Keep Thalomid in the blister pack until you take your daily dose.
- Swallow Thalomid capsules whole with water.
- Thalomid is taken 1 time each day, at least 1 hour after your evening meal. Bedtime is the preferred time to take Thalomid.
- Do not open or crush Thalomid capsules or handle them any more than needed.
- If powder from the Thalomid capsule comes in contact with your skin, wash the skin right away with soap and water.
- If powder from the Thalomid capsule comes in contact with the inside of your eyes, nose, and mouth, flush well with water.
- If you miss a dose of Thalomid and it has been less than 12 hours since your regular time, take it as soon as you remember. If it has been more than 12 hours, just skip your missed dose. Do not take 2 doses at the same time.
- If you take too much Thalomid, call your healthcare provider right away.
What should I avoid while taking Thalomid?
- See “What is the most important information I should know about Thalomid?”
- Females: Do not get pregnant and do not breastfeed while taking Thalomid.
- Males: Do not donate sperm.
- Do not share Thalomid with other people. It may cause birth defects and other serious problems.
- Do not donate blood while you take Thalomid, during any breaks (interruptions) in your treatment, and for 4 weeks after stopping Thalomid. If someone who is pregnant gets your donated blood, her baby may be exposed to Thalomid and may be born with birth defects.
- Thalomid can cause drowsiness and sleepiness. Avoid drinking alcohol, operating machinery, and driving a car when taking Thalomid. Avoid taking other medicines that may cause drowsiness without talking to your healthcare provider first.
What are the possible side effects of Thalomid?
Thalomid can cause serious side effects, including:
- See “What is the most important information I should know about Thalomid?”
- Drowsiness and sleepiness. See “What should I avoid while taking Thalomid?”
- Nerve damage. Nerve damage is common with Thalomid. If the nerve damage is severe, it may not go away. Stop taking Thalomid and call your healthcare provider right away if you have any of these early symptoms of nerve damage in your hands, legs, or feet:
- numbness
- tingling
- pain
- burning sensation
- Dizziness and decreased blood pressure when changing positions. Thalomid may cause a decrease in your blood pressure, and you may feel dizzy when you go from a lying down or sitting position to standing up. When changing positions, sit upright for a few minutes before standing to help prevent this.
- Decreased white blood cell count. Thalomid can cause decreased white blood cell counts, including neutrophils. Neutrophils are a type of white blood cell that is important in fighting bacterial infections. Your healthcare provider should check your white blood count before and regularly while you take Thalomid. If your neutrophils are too low you should not start Thalomid and if they are low during treatment, your dose of Thalomid may need to be changed.
- Decreased platelet count. Thalomid can cause decreased platelet counts. Your healthcare provider should check your platelet count before and regularly while you take Thalomid. If your platelets are too low, your healthcare provider may lower your dose, delay your dose, or completely stop treatment. Tell your healthcare provider if you have signs and symptoms of bleeding such as:
- small red or purple spots on your body
- unusual bleeding or bruising
- nosebleeds
- bright red or tar like stools
- vomiting or coughing up blood
- Increased HIV virus in the blood. If you are HIV positive, your healthcare provider should check your viral load after one month and three months of treatment, then every 3 months after that.
- Slow heartbeat (bradycardia). Tell your healthcare provider if you have a slow heartbeat, fainting, dizziness or shortness of breath.
- Severe skin reactions and severe allergic reactions. Severe skin reactions and severe allergic reactions can happen with Thalomid and may cause death.
Call your healthcare provider right away if you develop any of the following signs or symptoms during treatment with Thalomid:- a red, itchy, skin rash
- peeling of your skin or blisters
- severe itching
- feverGet emergency medical help right away if you develop any of the following signs or symptoms during treatment with Thalomid:
- swelling of your lips, mouth, tongue, or throat
- trouble breathing or swallowing
- raised red areas on your skin (hives)
- a very fast heartbeat
- you feel dizzy or faint
- Seizures. Tell your healthcare provider right away if you have a seizure while taking Thalomid.
- Tumor Lysis Syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure and sometimes death. Your healthcare provider may do blood tests to check you for TLS.
- Birth control risks. Certain birth control methods may pose a higher risk of serious side effects and should not be used in some females. These risks include severe decreased white blood cell counts, low platelet counts, and blood clots. Use of an intrauterine device (IUD) or implantable birth control may also increase your risk of infection or bleeding during insertion, removal or during use of the device.
Your healthcare provider may tell you to decrease your dose, temporarily stop or permanently stop taking Thalomid if you develop certain serious side effects during treatment with Thalomid.
The most common side effects of Thalomid for treatment of multiple myeloma include:
- tiredness
- decreased calcium levels
- swelling of the hands and feet
- constipation
- numbness or tingling
- shortness of breath
- muscle weakness
- low blood counts
- skin rash or peeling. See “Severe skin reactions and severe allergic reactions” above.
- confusion
- decreased appetite
- nausea
- anxiety
- decreased energy or strength
- tremor
- fever
- weight loss
- blood clots
- muscle twitching and cramping
- weight gain
- dizziness
- dry skin
The most common side effects of Thalomid for treatment of leprosy include:
- sleepiness
- rash
- headache
These are not all the possible side effects of Thalomid.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
General information about the safe and effective use of Thalomid
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take Thalomid for conditions for which it was not prescribed. Do not give Thalomid to other people, even if they have the same symptoms you have. It may harm them and may cause birth defects. You can ask your healthcare provider or pharmacist for information about Thalomid that is written for health professionals.
How should I store Thalomid?
- Store Thalomid at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect from light.
- Return any unused Thalomid to Celgene or your healthcare provider.
Keep Thalomid and all medicines out of the reach of children.
What are the ingredients in Thalomid?
Active ingredient: thalidomide
Inactive ingredients: pregelatinized starch and magnesium stearate.
The 50 mg capsule shell contains gelatin, titanium dioxide and black ink.
The 100 mg capsule shell contains black iron oxide, yellow iron oxide, titanium dioxide, gelatin, and black ink.
The 150 mg capsule shell contains FD&C blue #2, black iron oxide, yellow iron oxide, titanium dioxide, gelatin, and black and white ink.
The 200 mg capsule shell contains FD&C blue #2, titanium dioxide, gelatin, and white ink.
Label

SRC: NLM .