Subsys
Generic name: fentanyl (buccal/sublingual)
Brand names: Abstral, Fentora, Subsys
Drug class: Narcotic analgesics
Medically reviewed by A Ras MD.
What is Subsys?
Subsys is a strong prescription pain medicine that contains an opioid (narcotic) that is used to manage breakthrough pain in adults (18 years of age and older) with cancer who are already routinely taking other opioid pain medicines around-the-clock for cancer pain. Subsys is started only after you have been taking other opioid pain medicines and your body has become used to them ( you are opioid tolerant). Do not use Subsys if you are not opioid tolerant.
it is n opioid pain medicine that can put you at risk for overdose and death. Even if you take your dose correctly as prescribed you are at risk for opioid addiction, abuse, and misuse that can lead to death.
Description
SUBSYS (fentanyl sublingual spray) is an opioid agonist, available as a sublingual spray designed to deliver doses of 100, 200, 400, 600, 800, 1200 and 1600 mcg of fentanyl. The chemical name of fentanyl is N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]propanamide.
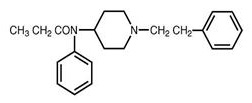
Fentanyl is a highly lipophilic compound (octanol-water partition coefficient at pH 7.4 is 860:1) that is freely soluble in ethanol and methanol and practically insoluble in water (1:40). The molecular weight of the free base is 336.47. The pKa is 8.4.
The inactive ingredients in SUBSYS include: dehydrated alcohol 63.6% (V/V), purified water, propylene glycol, xylitol, and L-menthol.
Mechanism of Action
Fentanyl is an opioid agonist whose principal therapeutic action is analgesia.
What is the most important information I should know about Subsys?
Do not use Subsys unless you are regularly using another opioid pain medicine around-the-clock for at least one week or longer for your cancer pain and your body is used to these medicines (this means that you are opioid tolerant). You can ask your healthcare provider if you are opioid tolerant.
Keep Subsys in a safe place away from children.
Get emergency help right away if:
- A child uses Subsys. Subsys can cause an overdose and death in any child who uses it.
- An adult who has not been prescribed Subsys uses it
- An adult who is not already taking opioids around-the-clock, uses Subsys
These are medical emergencies that can cause death.
- Get emergency help right away if you take too much SUBSYS (overdose) When you first start taking Subsys, when your dose is changed, or if you take too much (overdose), serious or life-threatening breathing problems that can lead to death may occur.
- Taking Subsys with other opioid medicines, that may make you sleepy, such as other pain medicines, anti-depressants, sleeping pills, anti-anxiety medicines, antihistamines, or tranquilizers, or with alcohol or street drugs can cause severe drowsiness, confusion, breathing problems, coma, and death.
- If you stop taking your around-the-clock opioid pain medicine for your cancer pain, you must stop using Subsys. You may no longer be opioid tolerant. Talk to your healthcare provider about how to treat your pain
- Never give anyone else your Subsys. They could die from taking it. Selling or giving away Subsys is against the law.
- Store Subsys securely, out of sight and reach of children, in a location not accessible by others, including visitors to the home.
- Subsys is available only through a program called the Transmucosal Immediate Release Fentanyl (TIRF) Risk Evaluation and Mitigation Strategy (REMS) Access program. To receive SUBSYS, you must:
- talk to your healthcare provider
- understand the benefits and risks of SUBSYS
- agree to all of the instructions
- sign the Patient-Prescriber Agreement form
- Subsys is only available at pharmacies that are part of the TIRF REMS Access program. Your healthcare provider will let you know the pharmacy closest to your home where you can have your Subsys prescription filled.
- Be very careful about taking other medicines that may make you sleepy, such as other pain medicines, anti-depressants, sleeping pills, anti-anxiety medicines, antihistamines, or tranquilizers.
- Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
Who should not take Subsys?
Do not take Subsys if:
- You are not opioid tolerant. Opioid tolerant means that you are already taking other opioid pain medicines around-the-clock for your cancer pain, and your body is used to these medicines.
- You have severe asthma, trouble breathing, or other lung problems.
- You have a bowel blockage or have narrowing of the stomach or intestines
- You have a short-term pain that you would expect to go away in a few days, such as:
- pain after surgery
- headache or migraine
- dental pain
- You have an allergy to any of the ingredients in Subsys:
- Active ingredient: fentanyl
- Inactive ingredients: dehydrated alcohol 63.6%, purified water, propylene glycol, xylitol, and L-menthol.
What should I tell my healthcare provider before taking Subsys?
Before taking Subsys, tell your healthcare provider if you have a history of:
- Trouble breathing or lung problems such as asthma, wheezing, or shortness of breath
- head injury, seizures
- liver, kidney, thyroid problems
- problems urinating
- pancreas or gallbladder problems
- abuse of street or prescription drugs, alcohol addiction
- mental problems including major depression, schizophrenia or hallucinations (seeing or hearing things that are not there)
- Slow heart rate or other heart problems
- Low blood pressure
Tell your healthcare provider if you are:
- Pregnant or planning to become pregnant. Prolonged use of Subsys during pregnancy can cause withdrawal symptoms in your newborn baby that could be life-threatening if not recognized and treated.
- Breastfeeding. Subsys passes into breast milk and may harm your baby.
- Taking prescription or over-the-counter medicines, vitamins, or herbal supplements. Taking Subsys with certain other medicines can cause serious side effects that could lead to death.
How should I take Subsys?
- Do not change your dose. Take Subsys exactly as prescribed by your healthcare provider.
- See the detailed Instructions for Use that come with Subsys for information about how to take Subsys.
- Use Subsys exactly as prescribed by your healthcare provider. Do not use more than 2 doses of Subsys for each episode of breakthrough cancer pain. You must wait four hours before treating a new episode of breakthrough pain with Subsys.
- Your healthcare provider will prescribe a starting dose of Subsys that may be different than other fentanyl containing medicines you may have been taking.
- Do not stop taking Subsys without talking to your healthcare provider.
- After you stop taking Subsys, see the instructions for use that come with Subsys for information about the right way to dispose of Subsys when no longer needed.
- Dispose of expired, unwanted or unused Subsys by following the “Disposing of Subsys” directions in the Instructions for Use. Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
What should I avoid while taking Subsys?
- DO NOT drive or operate heavy machinery, until you know how Subsys affects you. Subsys can make you sleepy, dizzy, or lightheaded.
- DO NOT drink alcohol or use prescription or over-the-counter medicines that contain alcohol. Using products containing alcohol during treatment with Subsys may cause you to overdose and die.
- DO NOT Switch from Subsys to other medicines that contain fentanyl without talking with your healthcare provider. The amount of fentanyl in a dose of Subsys is not the same as the amount of fentanyl in other medicines that contain fentanyl.
What are the possible side effects of Subsys?
The possible side effects of Subsys:
- Constipation, nausea, sleepiness, vomiting, tiredness, headache, dizziness, abdominal pain, weakness, anxiety, depression, rash, trouble sleeping, low red blood cell count, swelling of the arms, hands, legs, and feet. Call your healthcare provider if you have any of these symptoms and they are severe.
- Decreased blood pressure. This can make you feel dizzy or lightheaded if you get up too fast from sitting or lying down.
Get emergency medical help if you have:
- trouble breathing, shortness of breath, fast heartbeat, chest pain, swelling of your face, tongue, or throat, extreme drowsiness, light-headedness when changing positions, feeling faint, agitation, high body temperature, trouble walking, stiff muscles, or mental changes such as confusion.
These are not all the possible side effects of Subsys. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Subsys?
- Store at 20-25ºC (68-77ºF) with excursions permitted between 15º and 30ºC (59º to 86ºF) until ready to use. Do not use if the blister package has been opened.
- Keep Subsys in a safe place away from children.
Child Safety Kit
The Subsys Child Safety Kit contains important information on the storage and handling of Subsys.
The Subsys Child Safety Kit includes:
- a portable pouch (FIGURE A) and lock (FIGURE B) for you to keep a small supply of Subsys. Keep the rest of your Subsys in the locked storage space.
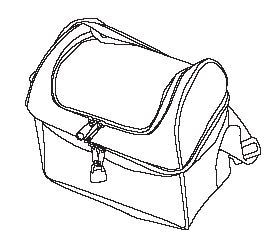
Figure A
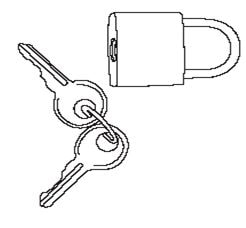
Figure B
- a package of cabinet and drawer child safety latches (FIGURE C) to secure the storage space where Subsys is kept at home.
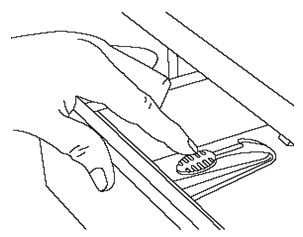
Figure C
- Keep the pouch locked and away from children. (See FIGURE C)
See the instructions for use that come with Subsys for instructions about disposing of Subsys properly.
What are the ingredients in Subsys?
Active ingredient: fentanyl
Label
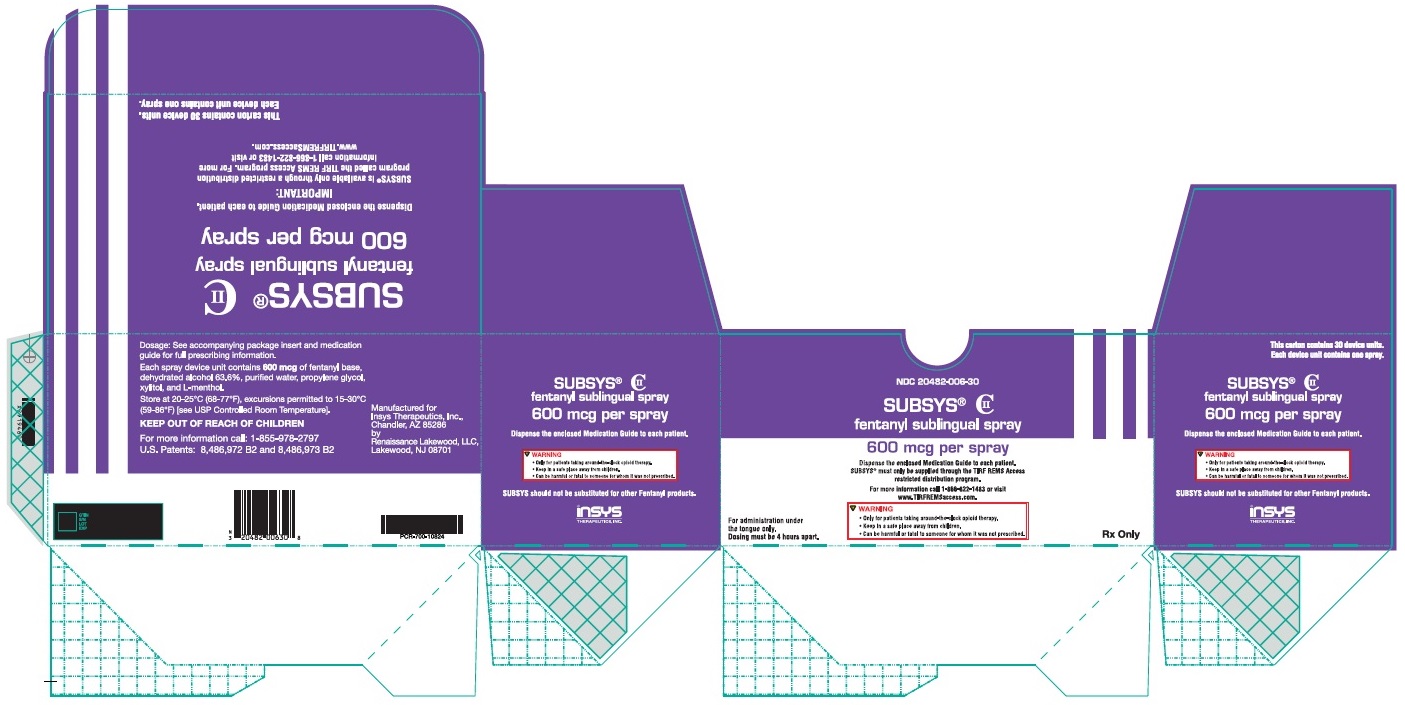
PRINCIPAL DISPLAY PANEL – NDC: 20482-001-10 – 100 MCG 10-CT CARTON LABEL

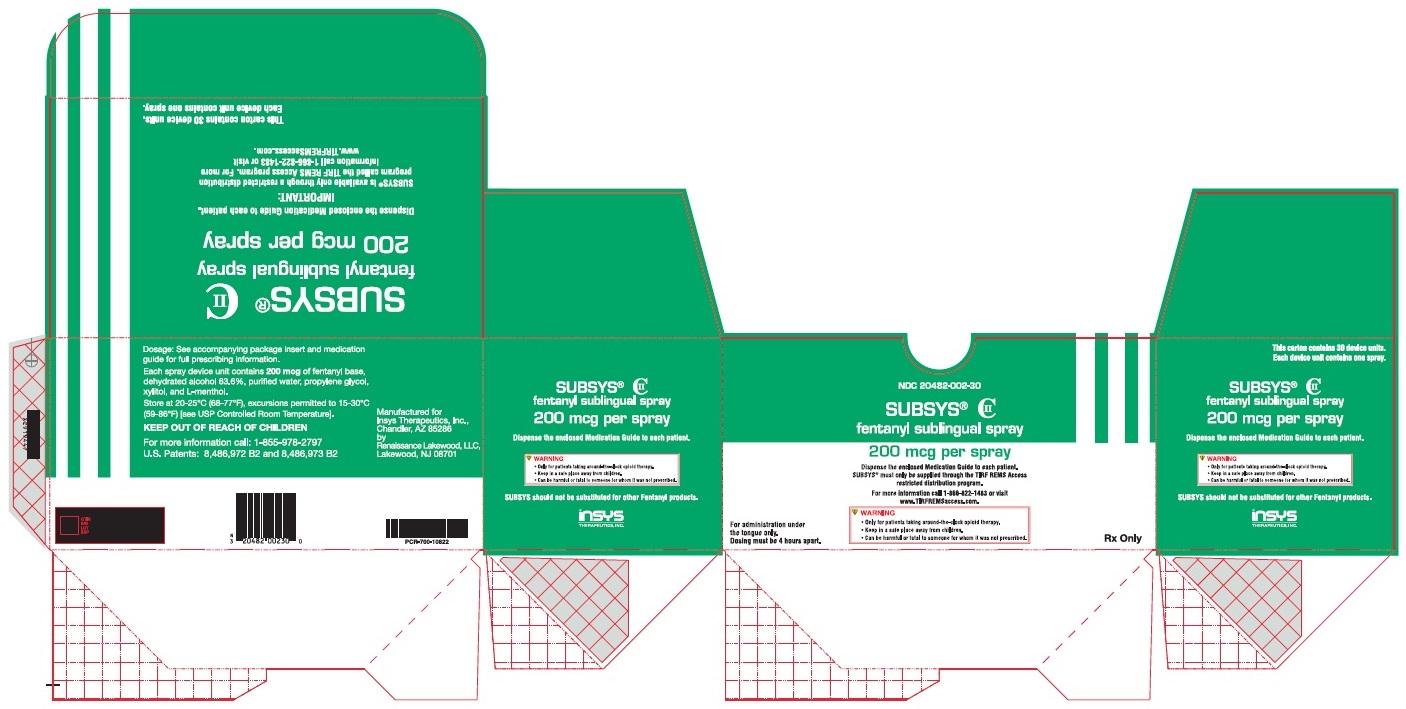
PRINCIPAL DISPLAY PANEL – NDC: 20482-002-30 – 200 MCG 30-CT CARTON LABEL
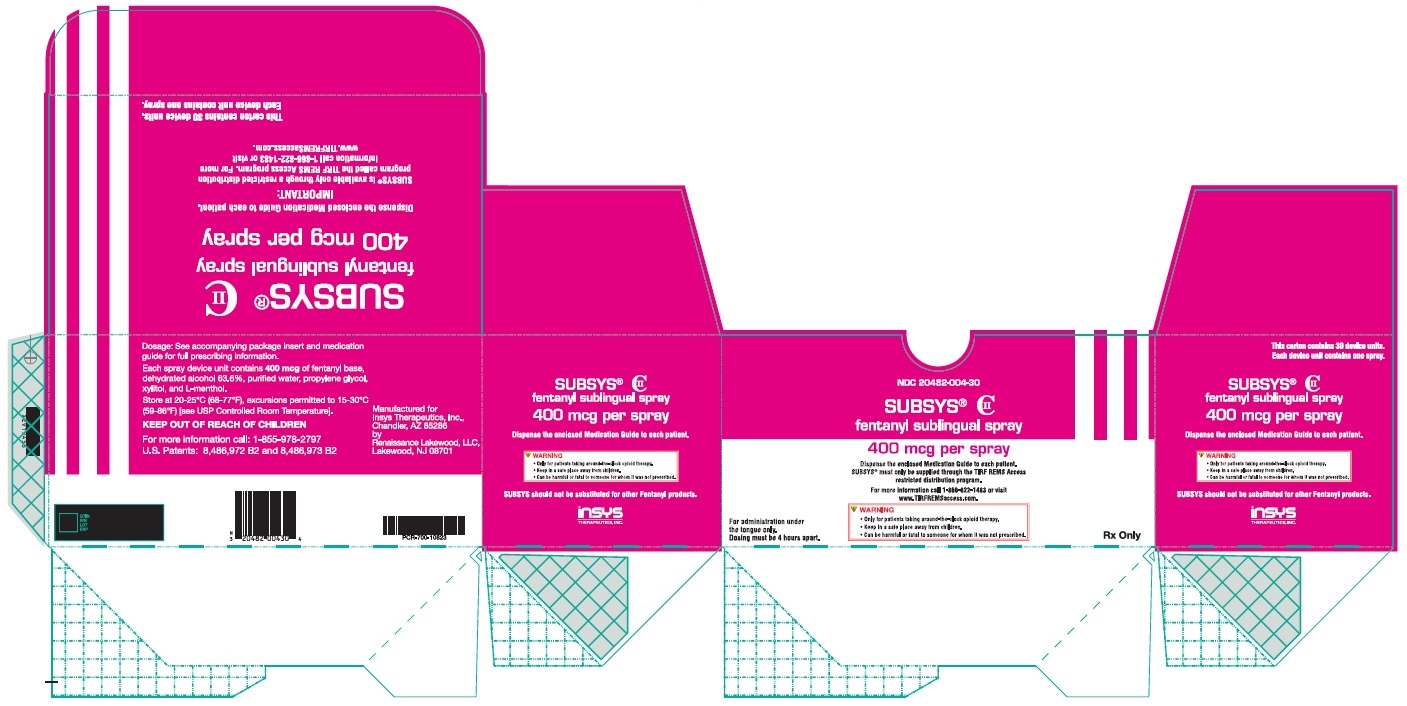
PRINCIPAL DISPLAY PANEL – NDC: 20482-004-30 – 400 MCG 30-CT CARTON LABEL
PRINCIPAL DISPLAY PANEL – NDC: 20482-006-30 – 600 MCG 30-CT CARTON LABEL