Reclast
Generic Name: zoledronic acid
Names of brands: Reclast
What is Reclast?
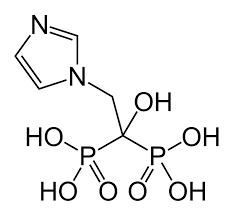
Reclast (zoledronic acid) is part of a family of drugs known as bisphosphonates. Reclast blocks the absorption of calcium out of bones.
Reclast can be used to treat Paget’s disease as well as osteoporosis for postmenopausal females.
Reclast s also employed for purposes that are not mentioned in this guide.¶
Warnings and Precautions
Don’t take Reclast without consulting your physician in the event that you’ve experienced reactions to an allergen such as Reclasts or similar medications like alendronate (Fosamax) or the etidronate (Didronel) or ibandronate (Boniva) or Pamidronate (Aredia) or risedronate (Actonel) as well as Tiludronate (Skelid).
Do not take Reclast before telling your physician in case you are expecting. It may cause harm to your unborn child. Make sure you use a safe method of birth control. Also, inform your physician if you develop a pregnancy while receiving treatment.
The doctor might suggest having a dental check-up to determine the need for preventive gum and tooth treatment prior to beginning treatment with Reclast. This is especially crucial in the case of cancer, are taking chemotherapy or steroids, or suffer from dental problems.
Certain patients who are taking medications like Reclast have suffered from jaw bone loss which is also known as osteonecrosis of the jaw. Signs of this disease could include jaw discomfort, swelling, numbness, gum inflammation, loose teeth, or slow healing after injuries or surgeries that involve the gums. It is possible to develop osteonecrosis in the jaw if you are suffering from cancer or have had treatment with radiation, chemotherapy, or steroids. Other ailments that are associated with osteonecrosis in the jaw are blood clotting disorders as well as anemia (low red blood cells) and previous dental problems.¶
In summary :
- Patients who are treated with Reclast injections shouldn’t be treated using the drug Reclast (r).
- Rehydrate patients in a timely manner with malignancy who have hypercalcemia prior to the administration of the injection of Reclast and observe electrolytes throughout treatment.
- It is possible that renal toxicity will be more severe in patients with impaired renal function. Avoid doses that are greater than four mg. Treatment for patients suffering from serious renal impairments is not advised. Check serum creatinine prior to every dose.
- Osteonecrosis in the jaw (ONJ) was identified. Dental exams for prevention must be conducted prior to starting the injection of zoledronic acid. Avoid any invasive dental procedures.
- A severe incapacitating joint, bone, or muscle pain could be experienced. Discontinue Reclast injection if severe symptoms occur.
- Diaphyseal and subtrochanteric fractures of the femur were reported among patients who receive bisphosphonate therapy. The fractures can occur following minor damage or without trauma. Examine patients suffering from discomfort in the groin or thigh to determine if there is a fracture in the femur. Beware of discontinuing medication for patients who may have an unusual femur fracture.
- Hypocalcemia: Make sure you are in good shape prior to initiating the injection of zoledronic acid. Be sure to adequately supply patients with vitamin D and calcium. Check the serum calcium levels closely in conjunction with the administration of other medications that can cause hypocalcemia. This will help avoid serious dangerous hypocalcemia.
- Reclast injections can cause harm to fetuses. Women should be warned of the danger to the fetus, and to take advantage of effective contraception.
Mechanism of Action
Reclast, a bisphosphonate that works primarily on bone. It acts as an inhibitor of osteoclasts that cause bone loss.
The action selective of bisphosphonates against bone is due to their affinity to the mineralized bone. When administered intravenously, zoledronic acids rapidly divide into the bone and are located more notably at areas of significant bone turnover. The principal molecular target of zoledronic acids in the osteoclast is the farnesyl pyrophosphate enzyme synthase. The long-lasting action of zoledronic acids is due to their specificity for binding to bone minerals.
Prior to receiving Reclast
It is not recommended to take Reclast in the event that you are allergic to Reclasts or similar medications like alendronate (Fosamax) and Etidronate (Didronel) Ibandronate (Boniva) and Pamidronate (Aredia) and risedronate (Actonel) and Tiludronate (Skelid).
Also, you should not get Reclast If you suffer from:
- Low levels of calcium in your blood.
- impaired kidney function; or
- If you are expecting or breastfeeding.
Zometa, as well as Reclast, are two distinct versions of Reclast. It is not recommended to be treated with Reclast when you’re already receiving Zometa. Before you receive the Reclast injection, inform your doctor that you are currently receiving Zometa.
Before you receive Reclast tell your physician if you suffer from:
- aspirin-sensitive asthma;
- A thyroid or parathyroid condition;
- malabsorption disorder (an inability to properly absorb nutrients and food correctly);
- an occurrence of surgical removal of a portion of your intestine
- bone cancer; or
- kidney disease.
The doctor might suggest having a dental check-up to ensure that you are receiving preventive dental and gum treatment prior to starting treatments with Reclast. This is particularly important in the case of cancer, are taking chemotherapy or steroids, or are suffering from poor dental health.
Some patients who are taking medications like Reclast have experienced jaw bone loss which is also known as osteonecrosis of the jaw. Signs of this disease could include jaw pain and swelling, numbness or gum infections, loose teeth, or slow healing following injuries or surgeries that involve the gums.
There is a higher chance to suffer from osteonecrosis of your jaw if you suffer from cancer or have had treatment using radiation, chemotherapy, or steroids. Other ailments that are associated with osteonecrosis in the jaw are blood clotting disorders as well as anemia (low red blood cells) and dental surgery or existing dental issues.
FDA classification for pregnancy D. This medication could harm an unborn child. Don’t take Reclast without informing your physician when you’re expecting. Make sure you are using a reliable method of birth control and inform your doctor if pregnant while receiving treatment. Reclast is a substance that can be found in breast milk and could harm nursing babies. Do not take Reclast without informing your doctor whether you are breastfeeding your baby.
How to take Reclast?
Reclast is administered by injection with a needle inserted in the vein. It is administered in a hospital or clinic or in a hospital. The medicine should be administered slowly, via an IV infusion. It can take up to 15 minutes to finish.
Reclast may be prescribed only every year. Follow the instructions of your physician.
Take at least two glasses of water just a few hours before your injection to prevent becoming dehydrated.
Your physician may ask you to take calcium or vitamin D supplements when you’re receiving treatment for Reclast. Follow your doctor’s advice regarding the strength and type of calcium that you need to take.
To make sure Reclast can help your condition and is not producing adverse unwanted side effects the blood of your patient needs to be checked frequently. Your kidney function could be a factor to be checked. It is crucial to do not to miss any appointments with your physician.
Information and usage
 Reclast is a bisphosphonate that is indicated for the treatment of
Reclast is a bisphosphonate that is indicated for the treatment of
- Hypercalcemia is a sign of malignancy.
- Patients suffering from multiple myeloma or patients with bone metastases documented from solid tumors, when combined with the standard antineoplastic treatment. Prostate cancer must have grown after treatment with at minimum one hormone treatment.
Limitations of Use The safety and effectiveness of Reclast injections have been questioned for their use for hyperparathyroidism or other non-tumor-related hypercalcemia.
Dosage and Administration
Important Administration Instructions
Reclast injections must be administered via an intravenous infusion for no under 15 minutes.
- Patients should be properly hydrated prior to the administration of Reclast.
- Drug products for the parent must be examined visually for discoloration or particulate matter ahead of administration if the solution and the container allow.
- Intravenous infusions are to follow by a 10mL normal saline flush from your intravenous line.
- The administration of acetaminophen after Reclast administration can decrease the risk of experiencing acute-phase symptoms of the reaction.
Management of Osteoporosis postmenopausal women
The suggested dosage is a 5-mg infusion every year, administered intravenously for no more than fifteen minutes.
Prevention of Osteoporosis in Postmenopausal Women
The recommended dosage is 5 mg of infusions administered once every two years, intravenously for no under 15 mins.
Osteoporosis in men
The suggested dosage is a 5-mg infusion, once per year, given intravenously for no more than fifteen minutes.
Treatment and Prevention of Glucocorticoid-Induced Osteoporosis
The recommended dosage is a 5-mg infusion every year, administered intravenously, not more than fifteen minutes.
Treatment for Paget’s Disease of Bone
The dose recommended is a 5-mg infusion. The time of infusion must not be shorter than 15 minutes if it is given at an infusion rate that is constant.
Re-treatment for Paget’s Disease
After a single treatment of Reclast in the case of Paget’s disease, an extended period of remission is observed. The specific re-treatment results are not available. However, re-treatment using Reclast could be considered for patients who have had a relapse, because of increases in serum alkaline-phosphatase levels, or in patients who were not able to achieve an improvement in the serum alkaline-phosphatase or in patients who have symptoms, as recommended by medical practices.
Laboratory Testing and Oral Examination prior to Administration
- Before administering every dose of Reclast take the serum creatinine level and creatinine clearance calculated based upon the actual body weight using the formula Cockcroft-Gault prior to every Reclast dose. Reclast is not recommended for patients with a clearance of less than 35 mL/min, and in patients who have evidence that they have acute kidney impairment. A dose of 5 mg of Reclast administered intravenously is suggested in patients with a clearance of greater than 35 mL/min, or more than. There are no safety or effectiveness studies to justify the adjustment of Reclast dose based upon the baseline renal function. So there is no dose adjustment needed in patients who have a creatinine clearance that is greater than or equal to 35 mL/min (see Contraindications (4) Warnings and Warnings (5.3)(5.3).
- An oral examination routinely must be conducted by the doctor prior to the beginning of Reclast treatments [see warnings and precautions (5.4)for more information].
Vitamin D and Vitamin D Supplementation with Vitamin D and Calcium
- Patients being treated for bone disease Paget about the significance of vitamin D and calcium supplementation to maintain the level of calcium in their blood, as well as on signs of hypocalcemia. Patients should consume 1500 mg daily of elemental calcium in doses divided (750 mg twice every day or 500 mg 3 times per daily) and 800 units of vitamin D daily, especially during the two weeks following Reclast treatment.
- Inform patients who are being treated for osteoporosis to take calcium supplements and vitamin D when their diet is insufficient. A minimum of 800-1000 International Units of vitamin D every day is suggested.
Technique of Administration
The Reclast infusion duration should not be under 15 minutes at a constant rate of infusion.
The infusion of intravenous fluid should follow by a 10-mL normal saline flush from an intravenous vein.
Infusion solutions for Reclast should not be permitted to be in contact with divalent cation or calcium-containing solutions. They must be administered only as a separate intravenous solution via an isolated infusion line vented.
Dosage and Strengths
- 5 mg in a 100 mL infuse solution.
If I don’t take the dose?
Consult your physician if you have missed a dose of Reclast.
If I consume too much?
Contact emergency medical assistance If you suspect you’ve taken excessive Reclast. The signs of an overdose could include tingling or numbness of your feet or hands and muscle stiffness and spasms in muscles that surround your eyes, irregular heartbeats, wheezing or difficulty breathing.
What to avoid?
Avoid performing any dental procedure while receiving treatment with Reclast. It could take longer than usual for you to heal.
Reclast side effects
Contact your doctor immediately If you experience any of the symptoms that indicate an allergic reaction, such as asthmatic hives, difficulty breathing, or swelling of your lips, face, or tongue. Contact your doctor immediately in case you experience any of these severe reactions:
- The frequency of urination is lower than normal, or none at all
- muscles spasms, numbness or tingly sensation (especially in the mouth);
- chills, fevers, and body aches. symptoms;
- pale skin, easy bruising rare weakness
- painful joint, bone, or muscle pain in the joint, bone, or muscle.
Less severe Reclast adverse effects could be:
- cough;
- chest pain
- Loss of appetite, nausea vomiting;
- diarrhea, constipation;
- fatigue, headache, dizziness sensation;
- lower blood pressure and swelling of your feet or legs;
- joint, muscle or bone discomfort or
- swelling or redness in the area where the needle was.
This isn’t a complete list of all side effects. other side effects could occur. Inform your physician about any unusual or unpleasant adverse reaction.
What other medications can impact Reclast?
Before taking Reclast to inform your doctor if taking any of the following medicines:
- A diuretic (water pill);
- an antibiotic like amikacin (Amikin) and an antibiotic such as amikacin (Amikin), (Garamycin) Kanamycin (Kantrex) and neomycin (Mycifradin Neo-Fradin, NeoTab, Neo-Fradin) netilmicin (Netromycin) and streptomycin tobramycin (Nebcin, Tobi);
- Other medications that could affect your kidneys like pentamidine (Nebupent) and tacrolimus (Prograf) amphotericin B (Fungizone, AmBisome, Amphotec, Abelcet), capreomycin (Capastat), and the drug rifampin (Rifadin, Rimactane, Rifater) vancomycin (Vancocin Vancoled) Acyclovir (Zovirax) Adefovir (Hepsera) Cidofovir (Vistide) as well foscarnet (Foscavir) as well as
- chemotherapy drugs like aldesleukin (Proleukin) Carmustine (BiCNU, Gliadel), Cisplatin (Platinol) as well as IFOSFamide (Ifex) and oxaliplatin (Eloxatin) and the drug plicamycin (Mithracin) and streptozocin (Zanosar) or thalidomide (Thalomid) and Tretinoin (Vesanoid).
The list below isn’t exhaustive and there are other medications that interfere with Reclast. Inform your doctor about every prescription and over-the-counter medicine you take. This includes minerals, vitamins, or herbal supplements, as well as medications prescribed by doctors. Do not begin taking any new medication without first talking to your physician.
How supplied

- 5 mg/100 mL
- Solution for Intravenous Infusion
Additional details
- The best efforts have been made to make sure that the information given is up-to-date, accurate. However, there is no guarantee in this regard. Information on drugs in this publication could be time-sensitive.
- A lack of warning on a particular drug or combination of drugs does not in any way taken to mean that the substance or combination is effective, safe, or appropriate for a particular patient. The information in this article is not intended to be comprehensive and covers all possible use, directions, precautions and warnings, interactions with other drugs as well as allergic reactions or other adverse reactions.
