Kalydeco
Generic name: ivacaftor
Drug class: CFTR potentiators
Medically reviewed by A Ras MD.
What is Kalydeco?
Kalydeco is a prescription medicine used for the treatment of cystic fibrosis (CF) in patients age 4 months and older who have at least one mutation in their CF gene that is responsive to Kalydeco.
It is not known if Kalydeco is safe and effective in children under 4 months of age.
Description
The active ingredient in KALYDECO tablets and oral granules is ivacaftor, a cystic fibrosis transmembrane conductance regulator potentiator, which has the following chemical name: N-(2,4-di-tert-butyl-5-hydroxyphenyl)-1,4-dihydro-4-oxoquinoline-3-carboxamide. Its molecular formula is C24H28N2O3 and its molecular weight is 392.49. Ivacaftor has the following structural formula:
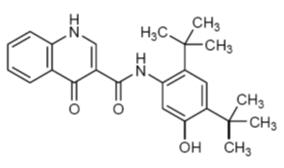
Ivacaftor is a white to off-white powder that is practically insoluble in water (<0.05 microgram/mL).
KALYDECO is available as a light blue, capsule shaped, film-coated tablet for oral administration containing 150 mg of ivacaftor. Each KALYDECO tablet contains 150 mg of ivacaftor and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The tablet film coat contains carnauba wax, FD&C Blue #2, PEG 3350, polyvinyl alcohol, talc, and titanium dioxide. The printing ink contains ammonium hydroxide, iron oxide black, propylene glycol, and shellac.
KALYDECO is also available as white to off-white granules for oral administration (sweetened but unflavored) and enclosed in a unit-dose packet containing 25 mg of ivacaftor, 50 mg of ivacaftor or 75 mg of ivacaftor. Each unit-dose packet of KALYDECO oral granules contains 25 mg of ivacaftor, 50 mg of ivacaftor or 75 mg of ivacaftor and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, mannitol, sucralose, and sodium lauryl sulfate.
Mechanism of Action
Ivacaftor is a potentiator of the CFTR protein. The CFTR protein is a chloride channel present at the surface of epithelial cells in multiple organs. Ivacaftor facilitates increased chloride transport by potentiating the channel open probability (or gating) of CFTR protein located at the cell surface. The overall level of ivacaftor-mediated CFTR chloride transport is dependent on the amount of CFTR protein at the cell surface and how responsive a particular mutant CFTR protein is to ivacaftor potentiation.
CFTR Chloride Transport Assay in Fisher Rat Thyroid (FRT) cells expressing mutant CFTR
The chloride transport response of mutant CFTR protein to ivacaftor was determined in Ussing chamber electrophysiology studies using a panel of FRT cell lines transfected with individual CFTR mutations. Ivacaftor increased chloride transport in FRT cells expressing CFTR mutations that result in CFTR protein being delivered to the cell surface.
The in vitro CFTR chloride transport response threshold was designated as a net increase of at least 10% of normal over baseline because it is predictive or reasonably expected to predict clinical benefit. For individual mutations, the magnitude of the net change over baseline in CFTR-mediated chloride transport in vitro is not correlated with the magnitude of clinical response. A patient must have at least one CFTR mutation responsive to ivacaftor to be indicated.
Who should not take Kalydeco?
Do not take Kalydeco if you take certain medicines or herbal supplements such as:
- the antibiotics rifampin (Rifamate, Rifater) or rifabutin (Mycobutin)
- seizure medications such as phenobarbital, carbamazepine (Tegretol, Carbatrol, Equetro) or phenytoin (Dilantin, Phenytek)
- St. John’s wort
Talk to your doctor before taking Kalydeco if you take any of the medicines or supplements listed above.
What should I tell my healthcare provider before taking Kalydeco?
Before you take Kalydeco, tell your doctor if you:
- have liver or kidney problems
- drink grapefruit juice, or eat grapefruit
- are pregnant or plan to become pregnant. It is not known if Kalydeco will harm your unborn baby. You and your doctor should decide if you will take Kalydeco while you are pregnant.
- are breastfeeding or planning to breastfeed. It is not known if Kalydeco passes into your breast milk. You and your doctor should decide if you will take Kalydeco while you are breastfeeding.
Kalydeco may affect the way other medicines work, and other medicines may affect how Kalydeco works.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements, as the dose of Kalydeco may need to be adjusted when taken with certain medications.
Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Especially tell your doctor if you take:
- antifungal medications such as ketoconazole (e.g., Nizoral), itraconazole (e.g., Sporanox), posaconazole (e.g., Noxafil), voriconazole (e.g., Vfend), or fluconazole (e.g., Diflucan)
- antibiotics such as telithromycin (e.g., Ketek), clarithromycin (e.g., Biaxin), or erythromycin (e.g., Ery-Tab)
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take Kalydeco?
- Take Kalydeco exactly as your doctor tells you to take it.
- Take your doses of Kalydeco 12 hours apart.
- If you miss a dose of Kalydeco and it is within 6 hours of when you usually take it, take your dose of Kalydeco as prescribed with fat-containing food as soon as possible.
- If you miss a dose of Kalydeco and it is more than 6 hours after the time you usually take it, skip that dose only and take the next dose when you usually take it. Do not take 2 doses at the same time to make up for your missed dose.
Kalydeco Tablets (ages 6 years and older):
- Always take Kalydeco tablets with food that contains fat. Examples of fat-containing foods include eggs, butter, peanut butter, cheese pizza, and whole-milk dairy products such as whole milk, cheese, and yogurt.
- Each Kalydeco box contains 4 individual blister cards.
- Each blister card contains 14 pills—7 morning doses and 7 evening doses.
- In the morning, unpeel the paper backing from a blister card to remove 1 Kalydeco tablet and take it with food that contains fat.
- In the evening, 12 hours later, open another blister card to remove 1 Kalydeco tablet and take it with food that contains fat.
- You may cut along the dotted line to separate your doses from the blister card.
Kalydeco Oral Granules (ages 4 months to under 6 years old):
- Hold the packet with cut line on top.
- Shake the packet gently to settle the Kalydeco granules.
- Tear or cut packet open along cut line.
- Carefully pour all of the Kalydeco granules in the packet into 1 teaspoon of soft food or liquid. Food or liquid should be at or below room temperature. Some examples of soft foods or liquids include puréed fruits or vegetables, yogurt, applesauce, water, breast milk, infant formula, milk, or juice.
- Mix the Kalydeco granules with food or liquid.
- After mixing, give Kalydeco within 1 hour. Make sure all medicine is taken.
- Give a child fat-containing food just before or just after the Kalydeco granules dose. Examples of fat-containing foods include eggs, butter, peanut butter, cheese pizza, and whole-milk dairy products such as whole milk, cheese, and yogurt.
What should I avoid while taking Kalydeco?
- Kalydeco can cause dizziness in some people who take it. Do not drive a car, use machinery or do anything that needs you to be alert until you know how Kalydeco affects you.
- You should avoid food containing grapefruit or Seville oranges while you are taking Kalydeco.
What are the possible side effects of Kalydeco?
Kalydeco can cause serious side effects.
High liver enzymes in the blood have been reported in patients receiving Kalydeco. Your doctor will do blood tests to check your liver:
- before you start Kalydeco
- every 3 months during your first year of taking Kalydeco
- every year while you are taking Kalydeco
For patients who have had high liver enzymes in the past, the doctor may do blood tests to check the liver more often.
Call your doctor right away if you have any of the following symptoms of liver problems:
- pain or discomfort in the upper right stomach (abdominal) area
- yellowing of your skin or the white part of your eyes
- loss of appetite
- nausea or vomiting
- dark, amber-colored urine
Abnormality of the eye lens (cataract) has been noted in some children and adolescents receiving Kalydeco.
Your doctor should perform eye examinations prior to and during treatment with Kalydeco to look for cataracts.
The most common side effects of Kalydeco include:
- headache
- upper respiratory tract infection (common cold), including:
- sore throat
- nasal or sinus congestion
- runny nose
- stomach (abdominal) pain
- diarrhea
- rash
- nausea
- dizziness
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Kalydeco. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Kalydeco
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Kalydeco for a condition for which it was not prescribed. Do not give Kalydeco to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information summarizes the most important information about Kalydeco. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Kalydeco that is written for health professionals.
For more information, go to www.kalydeco.com or call 1-877-752-5933.
How should I store Kalydeco?
- Store Kalydeco at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not use Kalydeco after the expiration date on the package.
Keep Kalydeco and all medicines out of the reach of children.
What are the ingredients in Kalydeco?
Active ingredient: ivacaftor
Inactive ingredients:
Kalydeco Tablets are light blue, film-coated, capsule-shaped tablets for oral administration and contain the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate.
The tablet film coat contains: carnauba wax, FD&C Blue #2, PEG 3350, polyvinyl alcohol, talc, and titanium dioxide.
The printing ink contains: ammonium hydroxide, iron oxide black, propylene glycol, and shellac.
Kalydeco Oral Granules are white to off-white granules for oral administration (sweetened but unflavored) and contain the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, mannitol, sucralose, and sodium lauryl sulfate.
Label
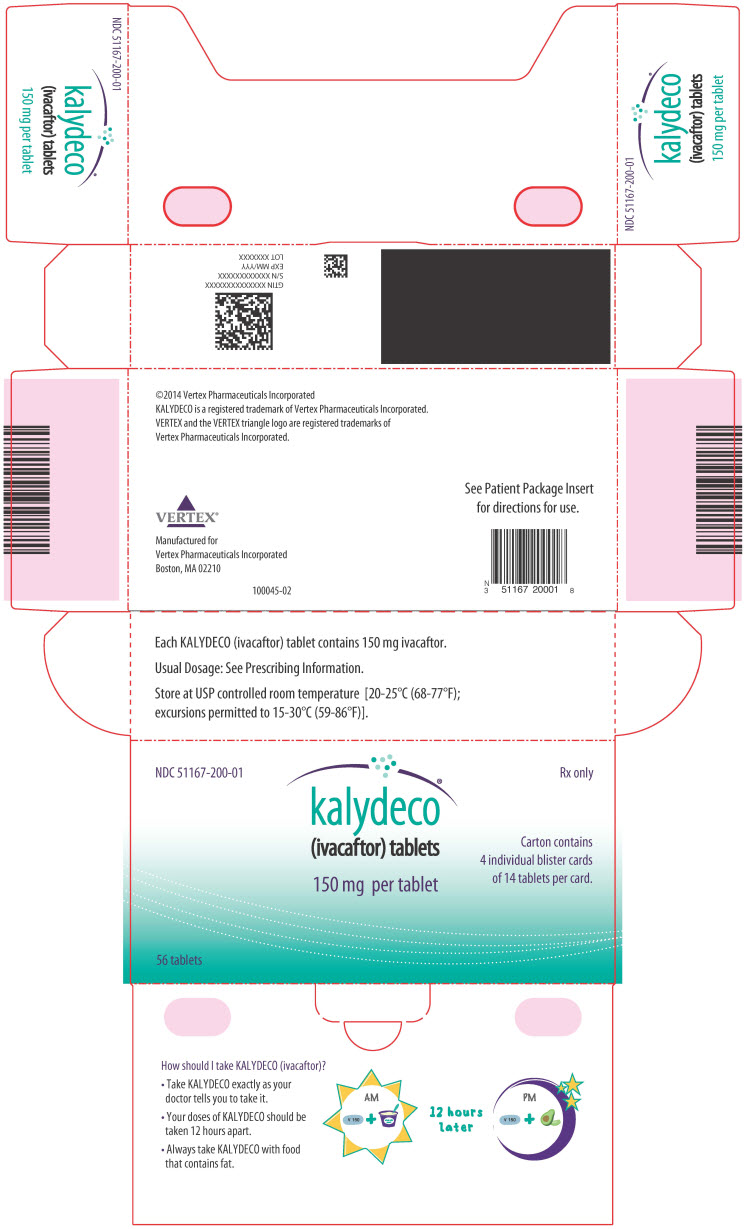
PRINCIPAL DISPLAY PANEL – 150 MG TABLET BLISTER CARD CARTON
- NDC 51167-200-01
Rx only - kalydeco®
(ivacaftor) tablets - 150 mg per tablet
- Carton contains
4 individual blister cards
of 14 tablets per card. - 56 tablets
SRC: NLM .
