Vfend
Generic name: voriconazole (oral/injection)
Drug class: Azole antifungals
Medically reviewed by A Ras MD.
What is Vfend?
Vfend is a prescription medicine used to treat certain serious fungal infections in your blood and body. These infections are called “aspergillosis,” “esophageal candidiasis,” “Scedosporium,” “Fusarium,” and “candidemia”.
It is not known if Vfend is safe and effective in children younger than 2 years old.
Description
VFEND (voriconazole), an azole antifungal agent is available as a lyophilized powder for solution for intravenous infusion. The structural formula is:
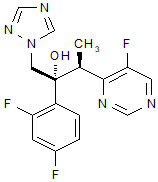
Voriconazole is designated chemically as (2R,3S)-2-(2, 4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol with an empirical formula of C16H14F3N5O and a molecular weight of 349.3.
Voriconazole drug substance is a white to light-colored powder.
VFEND I.V. is a white lyophilized powder containing nominally 200 mg voriconazole and 3200 mg sulfobutyl ether beta-cyclodextrin sodium in a 30 mL Type I clear glass vial.
VFEND I.V. is intended for administration by intravenous infusion. It is a single-dose, unpreserved product. Vials containing 200 mg lyophilized voriconazole are intended for reconstitution with Water for Injection to produce a solution containing 10 mg/mL VFEND and 160 mg/mL of sulfobutyl ether beta-cyclodextrin sodium. The resultant solution is further diluted prior to administration as an intravenous infusion
Who should not take Vfend?
Do not take Vfend if you:
- are allergic to voriconazole or any of the ingredients in Vfend. See the end of this guide for a complete list of ingredients in Vfend.
- are taking any of the following medicines:
Ask your healthcare provider or pharmacist if you are not sure if you are taking any of the medicines listed above.
Do not start taking a new medicine without talking to your healthcare provider or pharmacist.
What should I tell my healthcare provider before taking Vfend?
Before you take Vfend, tell your healthcare provider about all of your medical conditions, including if you:
- have or ever had heart disease, or an abnormal heart rate or rhythm. Your healthcare provider may order a test to check your heart (EKG) before starting Vfend.
- have low potassium levels, low magnesium levels, and low calcium levels. Your healthcare provider may do blood tests before starting and during treatment with Vfend.
- have liver or kidney problems. Your healthcare provider may do blood tests to make sure you can take Vfend.
- have trouble digesting dairy products, lactose (milk sugar), or regular table sugar. Vfend tablets contain lactose. Vfend liquid contains sucrose (table sugar).
- are pregnant or plan to become pregnant. Vfend can harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant. Women who can become pregnant should use effective birth control while taking Vfend. Talk to your healthcare provider about birth control methods that may be right for you.
- are breast-feeding or plan to breast-feed. It is not known if Vfend passes into breast milk. Talk to your healthcare provider about the best way to feed your baby if you take Vfend.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Vfend may affect the way other medicines work, and other medicines may affect how Vfend works.
Know what medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take Vfend?
- Vfend may be prescribed to you as:
- Vfend I.V. (intravenous infusion) or
- Vfend tablets or
- Vfend oral suspension
- Vfend I.V. will be given to you by a healthcare provider over 1 to 2 hours.
- Take Vfend tablets or oral suspension exactly as your healthcare provider tells you to.
- Take Vfend tablets or oral suspension at least 1 hour before or at least 1 hour after meals.
- Vfend oral suspension will be mixed for you by your pharmacist. Shake the bottle of Vfend oral suspension for 10 seconds each time before you use it.
- Only use the oral dispenser that comes with your Vfend oral suspension to administer your medicine.
- Do not mix Vfend oral suspension with any other medicine, flavored liquid, or syrup.
- See below for Instructions for use for Vfend oral suspension.
- If you take too much Vfend, call your healthcare provider or go to the nearest hospital emergency room.
What should I avoid while taking Vfend?
- You should not drive at night while taking Vfend. Vfend can cause changes in your vision such as blurring or sensitivity to light.
- Do not drive or operate machinery, or do other dangerous activities until you know how Vfend affects you.
- Avoid direct sunlight. Vfend can make your skin sensitive to the sun and the light from sunlamps and tanning beds. You could get a severe sunburn. Use sunscreen and wear a hat and clothes that cover your skin if you have to be in sunlight. Talk to your healthcare provider if you get sunburn.
What are the possible side effects of Vfend?
Vfend may cause serious side effects including:
- liver problems. Symptoms of liver problems may include:
- vision changes. Symptoms of vision changes may include:
- blurred vision
- changes in the way you see colors
- sensitivity to light (photophobia)
- serious heart problems. Vfend may cause changes in your heart rate or rhythm, including your heart stopping (cardiac arrest).
- allergic reactions. Symptoms of an allergic reaction may include:
- fever
- chest tightness
- nausea
- sweating
- trouble breathing
- itching
- feels like your heart is beating fast (tachycardia)
- feel faint
- skin rash
- kidney problems. Vfend may cause new or worse problems with kidney function, including kidney failure. Your healthcare provider should check your kidney function while you are taking Vfend. Your healthcare provider will decide if you can keep taking Vfend.
- serious skin reactions. Symptoms of serious skin reactions may include:
- rash or hives
- mouth sores
- blistering or peeling of your skin
- trouble swallowing or breathing
- adrenal gland problems:
- Vfend may cause reduced adrenal function (adrenal insufficiency).
- Vfend may cause overactive adrenal function (Cushing’s syndrome) when voriconazole is used at the same time with corticosteroids.Symptoms of adrenal insufficiency include:
- feeling tired
- nausea and vomiting
- abdominal pain
- lack of energy
- feeling dizzy or lightheaded
- weakness
- weight lossSymptoms of Cushing’s syndrome include:
- weight gain
- thinning skin
- excessive hair growth
- fatty hump between the shoulders (buffalo hump) and a rounded face (moon face)
- bruising easily
- excessive sweating
- darkening of the skin on the stomach, thighs, breasts, and arms
- high blood sugar
- bone problems. Vfend may cause weakening of bones and bone pain. Tell your healthcare provider if you have bone pain.
Call your healthcare provider or go to the nearest hospital emergency room right away if you have any of the symptoms listed above.
The most common side effects of Vfend in adults include:
- vision changes
- nausea
- hallucinations (seeing or hearing things that are not there)
- rash
- headache
- abnormal liver function tests
- chills
- vomiting
- fast heart beat (tachycardia)
- fever
The most common side effects of Vfend in children include:
- fever
- diarrhea
- low platelet counts
- abnormal liver function tests
- low blood calcium levels
- low blood phosphate levels
- vision changes
- rash
- stomach pain
- high blood pressure
- cough
- low blood pressure
- swelling in the arms and legs
- high blood sugar levels
- headache
- fast heart beat (tachycardia)
- nose bleeds
- low blood potassium levels
- Inflammation of mucous membranes
- hallucinations (seeing or hearing things that are not there)
- coughing up blood
- constipation
- low blood magnesium levels
- Fullness of the stomach area
- vomiting
- nausea
- upper respiratory tract infection
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Vfend.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Vfend
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Vfend for a condition for which it was not prescribed. Do not give Vfend to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Vfend that is written for health professionals.
How should I store Vfend?
- Store Vfend tablets and liquid at room temperature, 59°F to 86°F (15°C to 30°C). Do not refrigerate or freeze.
- Vfend suspension should be thrown away (discarded) after 14 days.
- Keep Vfend tablets and oral suspension in a tightly closed container.
- Safely throw away medicine that is out of date or no longer needed.
- Keep Vfend, as well as all other medicines, out of the reach of children.
What are the ingredients in Vfend?
Active ingredient: voriconazole
Inactive ingredients:
Vfend IV: sulfobutyl ether beta-cyclodextrin sodium
Vfend tablets: croscarmellose sodium, lactose monohydrate, magnesium stearate, povidone, pregelatinized starch, and a coating containing hypromellose, lactose monohydrate, titanium dioxide, and triacetin
Vfend oral suspension: anhydrous citric acid, colloidal silicon dioxide, natural orange flavor, sodium benzoate, sodium citrate dihydrate, sucrose, titanium dioxide, and xanthan gum
For more information, go to www.pfizer.com or call 1-800-438-1985
Instructions for use for Vfend oral suspension
Important information:
- Follow your healthcare provider’s instructions for the dose of Vfend to take.
- Ask your healthcare provider or pharmacist if you are not sure how to take Vfend.
- Vfend for oral suspension is a liquid form of Vfend. Your pharmacist will mix (reconstitute) the medicine before it is dispensed to you. If Vfend is still in powder form, do not use it. Return it to your pharmacist.
- Always use the oral dispenser provided with Vfend to make sure you measure the right amount of Vfend.
- Shake the closed bottle of mixed (reconstituted) oral suspension well for about 10 seconds before each use.
Each pack contains:
How to prepare the bottle and take Vfend:
1. Remove the child-resistant bottle cap by pushing down while twisting the cap to the left (counter-clockwise).
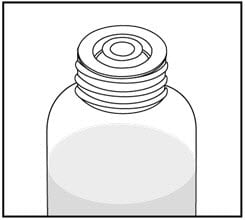
2. Push the bottle adapter firmly into the bottle (if your pharmacist has not already inserted the bottle adapter). If the bottle adapter is missing, contact your pharmacist.
Do not remove the bottle adapter after it is inserted.
3. Important: Bottle adapter must be fully inserted before use.
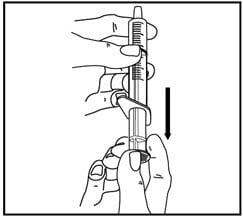
4. Pull back on the oral dispenser plunger to your prescribed dose.
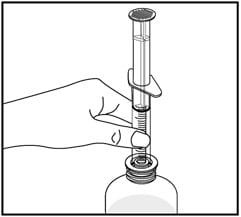
5. Insert the tip of the oral dispenser into the bottle adapter.
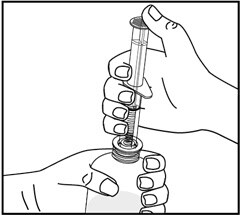
6. While holding the bottle with 1 hand, push down on the oral dispenser plunger with your other hand to push air into the bottle.
7. Turn the bottle upside down and slowly pull back on the oral dispenser plunger to withdraw your prescribed dose of medicine.
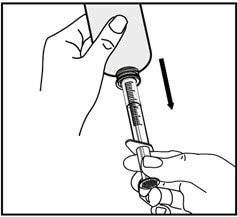
8. Turn the bottle back upright with the oral dispenser still in place. Remove the tip of the oral dispenser from the bottle adapter.
Place the tip of the oral dispenser in your mouth and point the tip of the oral dispenser towards the inside of the cheek.
Slowly push the plunger until all the medicine is given. Do not squirt the medicine out quickly. This may cause you to choke.
If the medicine is to be given to a child, keep your child in an upright position while giving the medicine.
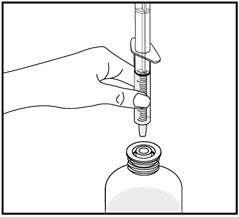
9. Screw the bottle cap back on the bottle tightly by turning the cap to the right (clockwise).
Do not remove the bottle adapter. The bottle cap will fit over it.
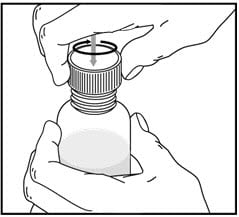
Rinse the oral dispenser after each use.
- Pull the plunger out of the oral dispenser and wash both parts with warm soapy water.
- Rinse both parts with water and allow to air dry after each use.
- After air drying, push the plunger back into the oral dispenser.
- Store the oral dispenser with Vfend oral suspension in a clean safe place.
How should I store Vfend oral suspension?
- Store Vfend oral suspension at room temperature between 59°F to 86°F (15°C to 30°C).
- Do not refrigerate or freeze.
- Keep the bottle cap tightly closed.
- Use Vfend oral suspension within 14 days after it has been mixed (reconstituted) by the pharmacist. The pharmacist will write the expiration date on the bottle label (the expiration date of the oral suspension is 14 days from the date it was mixed (reconstituted) by the pharmacist). Throw away (discard) any unused Vfend after the expiration date.
- Keep Vfend and all medicines out of the reach of children.
Label
PRINCIPAL DISPLAY PANEL – 200 MG VIAL LABEL
- NDC 0049-4190-01
- Vfend® I.V.
(voriconazole) for injection - 200 mg*
200 mg* of voriconazole - Not made with natural rubber latex
No Preservatives - Sterile Single-Dose Vial –
Discard Unused Portion
For Intravenous Infusion Only - Rx only
- PREMIERProRx®

SRC: NLM .
