Ixinity
Generic name: coagulation factor IX
Brand names: AlphaNine SD, Alprolix, BeneFIX, Idelvion, Ixinity, … show all 8 brands
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by A Ras MD.
What is Ixinity?
Ixinity is a medicine used to replace clotting factor (factor IX) that is missing in people with hemophilia B. Hemophilia B is also called congenital factor IX deficiency or Christmas disease. Hemophilia B is an inherited bleeding disorder that prevents clotting.
Your healthcare provider may give you Ixinity when you have surgery.
Description
IXINITY [coagulation factor IX (recombinant)] is a purified protein that has 415 amino acids. It has an amino acid sequence that is comparable to the Thr148 allelic form of plasma-derived factor IX. Coagulation factor IX (recombinant) is a single-chain glycoprotein with a molecular mass of about 55,000 Dalton that is secreted by a genetically engineered mammalian cell line derived from Chinese hamster ovary (CHO) cells. No human or animal proteins are added during any stage of manufacturing or formulation of IXINITY. The CHO cell line secretes recombinant factor IX into a defined cell culture medium that does not contain hormones. The recombinant factor IX is purified by a chromatography purification process. The process includes three validated steps for virus inactivation and removal, namely, solvent/detergent treatment, a chromatographic step, and nanofiltration. The process also includes a validated step to reduce the presence of CHO proteins in the final drug product.
IXINITY is formulated as a sterile, nonpyrogenic lyophilized powder to be reconstituted with Sterile Water for Injection for intravenous administration. It does not contain any preservatives and is available in single-use vials containing the labeled amount of factor IX activity, expressed in international units (IU). Each vial contains nominally 250, 500, 1000, 1500, 2000, or 3000 IU of recombinant coagulation factor IX. After reconstitution of the lyophilized powder, all dosage strengths yield a clear, colorless solution. The concentrations of excipients are:
| Excipient | Concentration |
| Histidine | 10 mM |
| Mannitol | 3% |
| Trehalose Dihydrate | 1% |
| Sodium Chloride | 66 mM |
| Polysorbate 80 | 0.0075% |
Mechanism of Action
Hemophilia B is a sex-linked hereditary disorder of blood coagulation caused by a deficiency in factor IX and results in bleeding into joints, muscles or internal organs, either spontaneously or as a result of accidental or surgical trauma. Treatment with IXINITY replaces factor IX, thereby enabling a temporary correction of the factor deficiency and correction of the bleeding tendencies.
Who should not use Ixinity?
You should not use Ixinity if you:
- Are allergic to hamsters
- Are allergic to any ingredients in Ixinity
Tell your healthcare provider if you are pregnant or breastfeeding because Ixinity may not be right for you.
What should I tell my healthcare provider before using Ixinity?
You should tell your healthcare provider if you:
- Have or have had any medical problems
- Take any medicines, including prescription and non-prescription medicines, such as over-the-counter medicines, supplements, or herbal remedies
- Have any allergies, including allergies to hamsters
- Are breastfeeding. It is not known if Ixinity passes into your milk and if it can harm your baby
- Are pregnant or planning to become pregnant. It is not known if Ixinity may harm your baby
- Have been told that you have inhibitors to factor IX (because Ixinity may not work for you)
How should I use Ixinity?
Ixinity is given directly into the bloodstream. Ixinity should be administered as ordered by your healthcare provider. You should be trained on how to do infusions by your healthcare provider or hemophilia treatment center. Many people with hemophilia B learn to infuse their Ixinity by themselves or with the help of a family member.
See the step-by-step instructions for use that come with Ixinity
Your healthcare provider will tell you how much Ixinity to use based on your weight, the severity of you hemophilia B, and where you are bleeding. You may have to have blood tests done after getting Ixinity to be sure that your blood level of factor IX is high enough to stop the bleeding. Call you healthcare provider right away if your bleeding does not stop after taking Ixinity.
What are the possible side effects of Ixinity?
Allergic reactions may occur with Ixinity. Call your healthcare provider or get emergency treatment right away if you get any of the following symptoms: rash, hives, itching, tightness of the throat, chest pain or tightness, difficulty breathing, lightheadedness, dizziness, nausea, or fainting.
Tell your healthcare provider about any side effect that bothers you or does not go away.
The most common side effect of Ixinity in clinical trials was headache.
These are not all the side effects possible with Ixinity. You can ask your healthcare provider for information that is written for healthcare professionals.
Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
General information about the safe and effective use of Ixinity
Your body may form inhibitors to factor IX. An inhibitor is part of the body’s immune system. If you form inhibitors, it may stop Ixinity from working properly. Consult with your healthcare provider to make sure you are carefully monitored with blood tests to check for the development of inhibitors to factor IX. Consult your doctor promptly if bleeding is not controlled with Ixinity as expected.
Medicines are sometimes prescribed for purposes other than those listed here. Do not use Ixinity for a condition for which it is not prescribed. Do not share Ixinity with other people, even if they have the same symptoms as you.
Resources available to patients
For information on patient assistance programs that may be available to you, please call our Ixinity Patient Care Center at 1-855-IXINITY (1-855-494-6489).
How should I store Ixinity?
250 IU strength only; store at 2 to 8°C (36 to 46°F). Do not freeze.
500, 1000, 1500, 2000, and 3000 IU strengths; store at 2 to 25°C (36 to 77°F). Do not freeze.
Do not use Ixinity after the expiration date printed on the label. Throw away any unused Ixinity and diluents after it reaches this date.
Reconstituted product (after mixing dry product with Sterile Water for Injection) must be used within 3 hours and cannot be stored or refrigerated. Discard any Ixinity left in the vial at the end of your infusion.
What are the ingredients in Ixinity?
Active ingredients: coagulation factor IX recombinant human
Inactive ingredients: water
What are the Ixinity dosage strengths?
Ixinity comes in vials containing six different dosage strengths: 250, 500, 1000, 1500, 2000 and 3000 international units (IU). The actual strength will be printed on the label of the vial and on the box. The six different strengths in the vials are color coded as follows:
| Color Code | Nominal Strength |
| Yellow | 250 IU |
| Blue | 500 IU |
| Green | 1000 IU |
| Orange | 1500 IU |
| Red | 2000 IU |
| Brown | 3000 IU |
Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider.
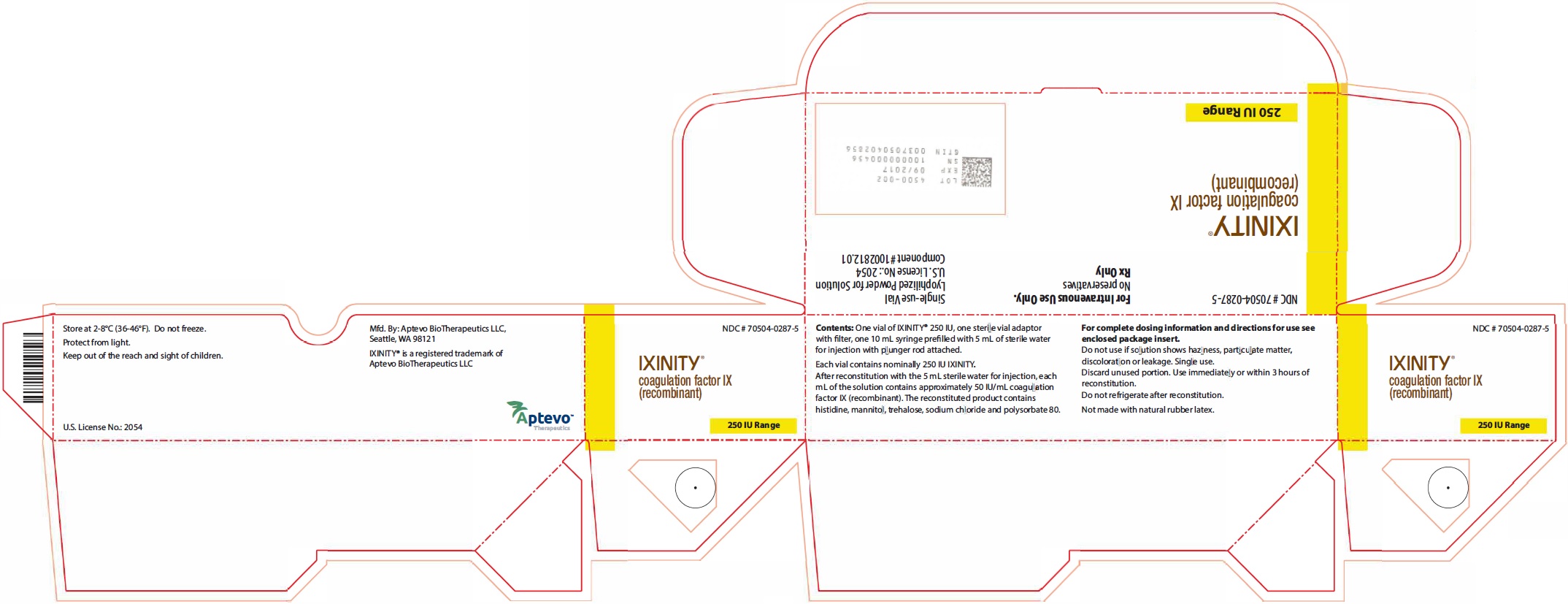
Label
PRINCIPAL DISPLAY PANEL – NDC: 70504-0275-1 – 250 IU SINGLE-USE VIAL LABEL

PRINCIPAL DISPLAY PANEL – NDC: 70504-0287-5 – 250 IU KIT CARTON
PRINCIPAL DISPLAY PANEL – NDC: 70504-0270-1 – 500 IU SINGLE-USE VIAL LABEL

