BeneFix
Generic name: coagulation factor IX
Brand names: AlphaNine SD, Alprolix, BeneFIX, Idelvion, Ixinity, … show all 8 brands
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by A Ras MD.
What is BeneFix?
BeneFix is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital factor IX deficiency or Christmas disease. BeneFix is not used to treat hemophilia A.
Description
BeneFIX, Coagulation Factor IX (Recombinant), is a purified protein produced by recombinant DNA technology. The product is formulated as a sterile, non-pyrogenic, lyophilized powder preparation intended to be reconstituted for intravenous injection. It is available in single-use vials containing the labeled amount of factor IX activity, expressed in International Units (IU). Each vial contains nominally 250, 500, 1000, 2000, or 3000 IU of recombinant coagulation factor IX. The potency (in IU) is determined using an in vitro one-stage clotting assay against the World Health Organization (WHO) International Standard for Factor IX concentrate. One IU is the amount of factor IX activity present in 1 mL of pooled, normal human plasma. After reconstitution of the lyophilized drug product, the concentrations of excipients are 0.234% sodium chloride, 8 mM L-histidine, 0.8% sucrose, 208 mM glycine, 0.004% polysorbate 80. The specific activity of BeneFIX is greater than or equal to 200 IU per milligram of protein. BeneFIX contains no preservatives and all dosage strengths yield a clear, colorless solution upon reconstitution.
Coagulation factor IX is the active ingredient in BeneFIX. It has a primary amino acid sequence that is identical to the Ala148 allelic form of human factor IX, and has structural and functional characteristics similar to those of endogenous factor IX.
BeneFIX is not derived from human blood. It is produced by a genetically engineered Chinese hamster ovary (CHO) cell line that is extensively characterized. No additives of animal or human origin are used during the cell culture, purification, and formulation processes of BeneFIX. The stored cell banks are free of human blood or plasma products. The CHO cell line secretes recombinant factor IX into a defined cell culture medium, and the recombinant factor IX is purified by a four-step chromatography purification process that does not require a monoclonal antibody step. The process also includes a membrane nanofiltration step that has the ability to retain molecules with apparent molecular weights >70,000 Da (such as large proteins and viral particles). BeneFIX is a single component by SDS-polyacrylamide gel electrophoresis evaluation.
Mechanism of Action
BeneFIX temporarily replaces the missing clotting factor IX that is needed for effective hemostasis.
What should I tell my healthcare provider before using BeneFix?
Tell your doctor and pharmacist about all of the medicines you take, including all prescription and non-prescription medicines, such as over-the-counter medicines, supplements, or herbal medicines.
Tell your doctor about all of your medical conditions, including if you:
- are pregnant or planning to become pregnant. It is not known if BeneFix may harm your unborn baby.
- are breastfeeding. It is not known if BeneFix passes into the milk and if it can harm your baby.
How should I use BeneFix?
The initial administrations of BeneFix should be administered under proper medical supervision, where proper medical care for severe allergic reactions could be provided.
See the step-by-step instructions for infusing BeneFix that come with BeneFix. You should always follow the specific instructions given by your doctor. The steps listed below are general guidelines for using BeneFix. If you are unsure of the procedures, please call your doctor or pharmacist before using.
Call your doctor right away if bleeding is not controlled after using BeneFix.
Your doctor will prescribe the dose that you should take.
Your doctor may need to test your blood from time to time.
BeneFix should not be administered by continuous infusion.
What if I take too much BeneFix?
Call your doctor if you take too much BeneFix.
What are the possible side effects of BeneFix?
Allergic reactions may occur with BeneFix. Call your doctor or get emergency treatment right away if you have any of the following symptoms:
- wheezing
- difficulty breathing
- chest tightness
- turning blue (look at lips and gums)
- fast heartbeat
- swelling of the face
- faintness
- rash
- hives
Your body can also make antibodies, called “inhibitors,” against BeneFix, which may stop BeneFix from working properly.
Some common side effects of BeneFix are nausea, injection site reaction, injection site pain, headache, dizziness and rash.
BeneFix may increase the risk of thromboembolism (abnormal blood clots) in your body if you have risk factors for developing blood clots, including an indwelling venous catheter through which BeneFix is given by continuous infusion. There have been reports of severe blood clotting events, including life-threatening blood clots in critically ill neonates, while receiving continuous-infusion BeneFix through a central venous catheter. The safety and efficacy of BeneFix administration by continuous infusion have not been established.
These are not all the possible side effects of BeneFix.
Tell your doctor about any side effect that bothers you or that does not go away.
General information about the safe and effective use of BeneFix
Medicines are sometimes prescribed for purposes other than those listed here. Do not use BeneFix for a condition for which it was not prescribed. Do not share BeneFix with other people, even if they have the same symptoms that you have.
This Patient Leaflet summarizes the most important information about BeneFix. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about BeneFix that was written for healthcare professionals.
How should I store BeneFix?
Do not freeze BeneFix kit.
BeneFix kit can be stored at room temperature (below 86°F) or under refrigeration.
Throw away any unused BeneFix and diluent after the expiration date indicated on the label.
Freezing should be avoided to prevent damage to the pre-filled diluent syringe.
BeneFix does not contain a preservative. After reconstituting BeneFix, you can store it at room temperature for up to 3 hours. If you have not used it in 3 hours, throw it away.
Do not use BeneFix if the reconstituted solution is not clear and colorless.
What are the ingredients in BeneFix?
Active ingredient: coagulation factor ix recombinant human
Inactive ingredients: histidine, glycine, sucrose, polysorbate 80
Diluent injection, solution: Sodium chloride, water
Alcohol wipe: isopropyl alcohol, water
Instructions for use
BeneFIX/ BEN-uh-fiks/
[Coagulation Factor IX (Recombinant)]
BeneFIX is supplied as a powder. Before it can be infused in your vein (intravenous injection), you must reconstitute the powder by mixing it with the liquid diluent supplied. The liquid diluent is 0.234% sodium chloride. BeneFIX should be reconstituted and infused using the infusion set, diluent, syringe, and adapter provided in this kit, and by following the directions below.
Reconstitution
Always wash your hands before performing the following steps. Try to keep everything clean and germ-free while you are reconstituting BeneFIX. Once you open the vials, you should finish reconstituting BeneFIX as soon as possible. This will help keep the infusion set materials germ-free.
Note: If you use more than one vial of BeneFIX per infusion, reconstitute each vial according to steps 1 through 13.
1. If refrigerated, let the vial of BeneFIX and the pre-filled diluent syringe reach room temperature.
2. Remove the plastic flip-top cap from the BeneFIX vial to show the center part of the rubber stopper.
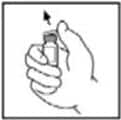
3. Wipe the top of the vial with the alcohol swab provided, or use another antiseptic solution, and allow to dry. After cleaning, do not touch the rubber stopper with your hand or allow it to touch any surface.
4. Peel back the cover from the clear plastic vial adapter package. Do not remove the adapter from the package.
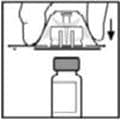
5. Place the vial on a flat surface. While holding the adapter in the package, place the vial adapter over the vial. Press down firmly on the package until the adapter snaps into place on top of the vial, with the adapter spike penetrating the vial stopper.
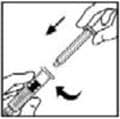
6. Grasp the plunger rod as shown in the picture below. Do not touch the shaft of the plunger rod. Attach the threaded end of the plunger rod to the diluent syringe plunger by pushing and turning firmly.
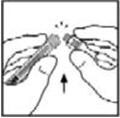
7. Break off the tamper-resistant, plastic-tip cap from the diluent syringe by snapping the perforation of the cap. Do not touch the inside of the cap or the syringe tip. The diluent syringe may need to be recapped (if reconstituted BeneFIX is not used immediately), so place the cap on its tip on a clean surface in a spot where it will stay clean.
8. Lift the package away from the adapter and discard the package.
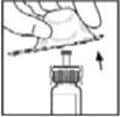
9. Place the vial on a flat surface. Connect the diluent syringe to the vial adapter by inserting the tip of the syringe into the adapter opening while firmly pushing and turning the syringe clockwise until the connection is secured.
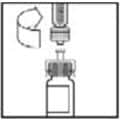
10. Slowly push the plunger rod to inject all the diluent into the BeneFIX vial.
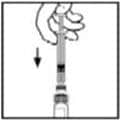
11. With the syringe still connected to the adapter, gently swirl the contents of the vial until the powder is dissolved.
Look at the final solution before infusing it. The solution should be clear to colorless. If it is not, throw away the solution and use a new kit.
12. Make sure the syringe plunger rod is still fully pressed down, then turn over the vial. Slowly pull the solution into the syringe. Turn the syringe upward again and remove any air bubbles by gently tapping the syringe with your finger and slowly pushing air out of the syringe.
If you reconstituted more than one vial of BeneFIX, remove the diluent syringe from the vial adapter and leave the vial adapter attached to the vial. Quickly attach a separate large luer lock syringe and pull the reconstituted solution as instructed above. Repeat this procedure with each vial in turn. Do not detach the diluent syringes or the large luer lock syringe until you are ready to attach the large luer lock syringe to the next vial adapter.
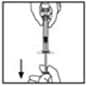
13. Remove the syringe from the vial adapter by gently pulling and turning the syringe counter-clockwise. Throw away the vial with the adapter attached.
If you are not using the solution right away, you should carefully replace the syringe cap. Do not touch the syringe tip or the inside of the cap.
BeneFIX should be infused within 3 hours after reconstitution. The reconstituted solution may be stored at room temperature prior to infusion.
Infusion (Intravenous Injection)
Continuous infusion is not an approved way to administer BeneFIX.
Your doctor or healthcare professional should teach you how to infuse BeneFIX. Once you learn how to self-infuse, you can follow the instructions in this insert.
1. Attach the syringe to the luer end of the provided infusion set tubing.
2. Apply a tourniquet and prepare the injection site by wiping the skin well with an alcohol swab provided in the kit.
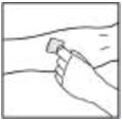
3. Insert the butterfly needle of the infusion set tubing into your vein as instructed by your doctor or healthcare provider. Remove the tourniquet. Infuse the reconstituted BeneFIX product over several minutes. Your comfort level should determine the rate of infusion.
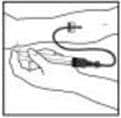
Clumping of red blood cells in the tubing/syringe has been reported with the administration of BeneFIX. No adverse events have been reported in association with this observation. To minimize the possibility of clumping it is important to limit the amount of blood entering the tubing. Blood should not enter the syringe.
Note: If red blood cell clumping is observed in the tubing or syringe, discard all material (tubing, syringe and BeneFIX solution) and continue administration with a new package.
4. After infusing BeneFIX, remove the infusion set and discard. The amount of drug product left in the infusion set will not affect your treatment. Dispose of all unused solution, the empty vial(s), and the used needles and syringes in an appropriate container used for throwing away waste that might hurt others if not handled properly.
It is a good idea to record the lot number from the BeneFIX vial label every time you use BeneFIX. You can use the peel-off label found on the vial to record the lot number.
If you have any questions or concerns about BeneFIX, ask your doctor or healthcare provider.
Label
PRINCIPAL DISPLAY PANEL – 1000 IU VIAL LABEL
- 1000 IU Range
- BeneFix®
Coagulation Factor IX (Recombinant)
Room Temperature Storage - No Preservatives
Single Use - Pfizer
Rx only - For Factor IX
Replacement
PRINCIPAL DISPLAY PANEL – 5 ML SYRINGE LABEL – KIT 58394-635
- 0.234% Sodium
Chloride Diluent
5 mL - Disposable syringe for
drug diluent use with BeneFix®
Coagulation Factor IX (Recombinant) - Sterile, nonpyrogenic. Contains no
preservatives. - Manufactured for
Wyeth Pharmaceuticals LLC
A subsidiary of Pfizer Inc
Philadelphia, PA 19101
Manufactured by
Vetter Pharma-Fertigung GmbH & Co. KG
Schuetzenstrasse 87, 99-101
88212 Ravensburg, Germany
or
Vetter Pharma-Fertigung GmbH & Co. KG
Eisenbahnstrasse 2-4
88085 Langenargen, Germany
US Govt. License No. 3 - Rx only
- LOT:
EXP.:
PAA133670

