Idelvion
Generic name: coagulation factor IX
Brand names: AlphaNine SD, Alprolix, BeneFIX, Idelvion, Ixinity, … show all 8 brands
Drug class: Miscellaneous coagulation modifiers
y reviewed by A Ras MD.
What is Idelvion used for?
Idelvion is a prescription medicine that is used to treat hemophilia. It is used to treat or prevent bleeding.
Description
IDELVION, Coagulation Factor IX (Recombinant), Albumin Fusion Protein (rIX-FP) is a sterile, non-pyrogenic, lyophilized powder to be reconstituted with sterile Water for Injection (sWFI) for intravenous administration. IDELVION is available in single-dose vials containing nominally 250, 500, 1000, 2000, or 3500 IU of Factor IX formulated with sodium citrate, polysorbate 80, mannitol and sucrose. The actual amount of Factor IX activity in IU is labeled on each vial. After reconstitution of the lyophilized powder, all dosage strengths yield a clear, yellow to colorless solution. IDELVION contains no preservatives.
The active ingredient in IDELVION, recombinant human coagulation Factor IX albumin fusion protein, is a purified protein produced by recombinant DNA technology. It is generated by the genetic fusion of recombinant albumin to recombinant coagulation Factor IX. The genetic fusion of the cDNA of human albumin to the cDNA of human coagulation Factor IX enables the gene product to be expressed as a single recombinant protein designated as rIX-FP.
The Factor IX portion of IDELVION is identical to the Thr148 allelic form of human plasma-derived Factor IX. The cleavable linker between the Factor IX and albumin moieties is derived from the endogenous activation peptide in native Factor IX. rIX-FP remains intact in the circulation until Factor IX is activated, whereupon albumin is cleaved from Factor IX, releasing activated Factor IX (FIXa) when it is needed for coagulation.
IDELVION is manufactured without the addition of proteins derived from human or animal source materials. IDELVION is a glycoprotein consisting of 1018 amino acids secreted by a genetically engineered Chinese hamster ovary (CHO) cell line. The CHO cell line secretes rIX-FP into a defined cell culture medium and the rIX-FP protein is purified by a process that does not require the use of a monoclonal antibody reagent. The manufacturing process incorporates three validated virus clearance steps, including virus inactivation by solvent/detergent treatment and virus removal by filtration.
The potency expressed in International Units is determined using an in vitro aPTT-based one-stage clotting assay against CSL Behring’s manufacturing reference standard. This internal potency standard has been calibrated against the World Health Organization (WHO) International Standard for Factor IX concentrate by a one-stage clotting assay using synthetic silica and synthetic phospholipid-based reagents.
Mechanism of Action
IDELVION is a recombinant protein that temporarily replaces the missing coagulation Factor IX needed for effective hemostasis. IDELVION is comprised of genetically fused recombinant coagulation Factor IX and recombinant albumin. Fusion with recombinant albumin extends the half-life of Factor IX
Before taking Idelvion, tell your doctor:
- If you are allergic to Idelvion; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you are allergic to hamsters, talk with the doctor.
This medicine may interact with other drugs or health problems.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take Idelvion with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take Idelvion?
- Tell all of your health care providers that you take Idelvion. This includes your doctors, nurses, pharmacists, and dentists.
- Allergic side effects may rarely happen.
- Blood clots have happened with Idelvion. Tell your doctor if you have ever had a blood clot. Talk with your doctor.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- Call the doctor right away if the normal dose does not work as well.
- Talk with the doctor before you travel. You will need to bring enough of Idelvion for use during travel.
- Tell your doctor if you are pregnant or plan on getting pregnant. You will need to talk about the benefits and risks of using Idelvion while you are pregnant.
- Tell your doctor if you are breast-feeding. You will need to talk about any risks to your baby.
How is Idelvion best taken?
Use Idelvion as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- It is given as a shot into a vein.
- This medicine may be given at home.
- If you will be giving yourself the shot, your doctor or nurse will teach you how to give the shot.
- Wash your hands before and after use.
- This medicine needs to be mixed before use. Follow how to mix as you were told by the doctor.
- Do not shake.
- Use within 4 hours of making.
- Do not use if the solution is cloudy, leaking, or has particles.
- Do not use if solution changes color.
- Throw away needles in a needle/sharp disposal box. Do not reuse needles or other items. When the box is full, follow all local rules for getting rid of it. Talk with a doctor or pharmacist if you have any questions.
What do I do if I miss a dose?
- Call your doctor to find out what to do.
What are the side effects of Idelvion that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of kidney problems like unable to pass urine, change in how much urine is passed, blood in the urine, or a big weight gain.
- Dizziness or passing out.
- Feeling confused.
- Weakness on 1 side of the body, trouble speaking or thinking, change in balance, drooping on one side of the face, or blurred eyesight.
- Call your doctor right away if you have signs of a blood clot like chest pain or pressure; coughing up blood; shortness of breath; swelling, warmth, numbness, change of color, or pain in a leg or arm; or trouble speaking or swallowing.
What are some other side effects of Idelvion?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Headache.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out Idelvion?
- Store at room temperature or in a refrigerator. Do not freeze.
- Store in the original container to protect from light.
- After mixing, do not refrigerate.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
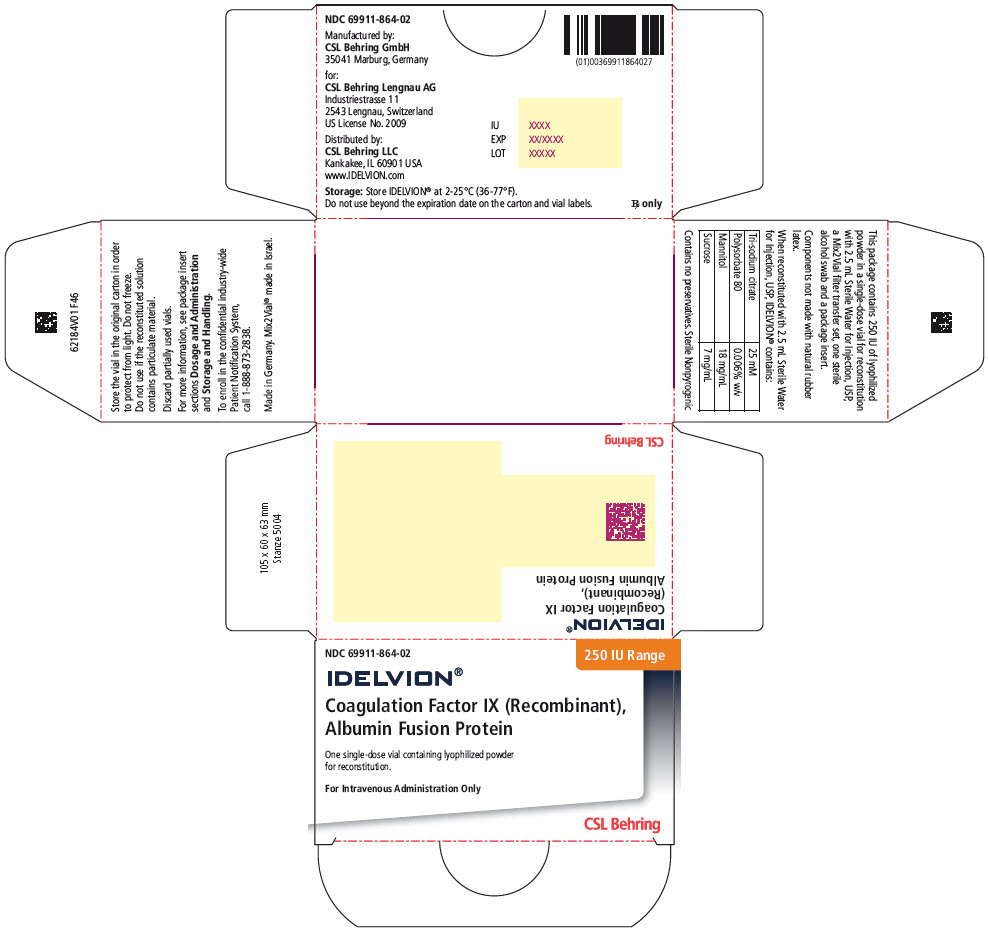
PRINCIPAL DISPLAY PANEL – 250 IU RANGE KIT CARTON
- NDC 69911-864-02
- 250 IU Range
- IDELVION®
- Coagulation Factor IX (Recombinant),
Albumin Fusion Protein - One single-use vial containing lyophilized powder
for reconstitution. - For Intravenous Administration Only
- CSL Behring
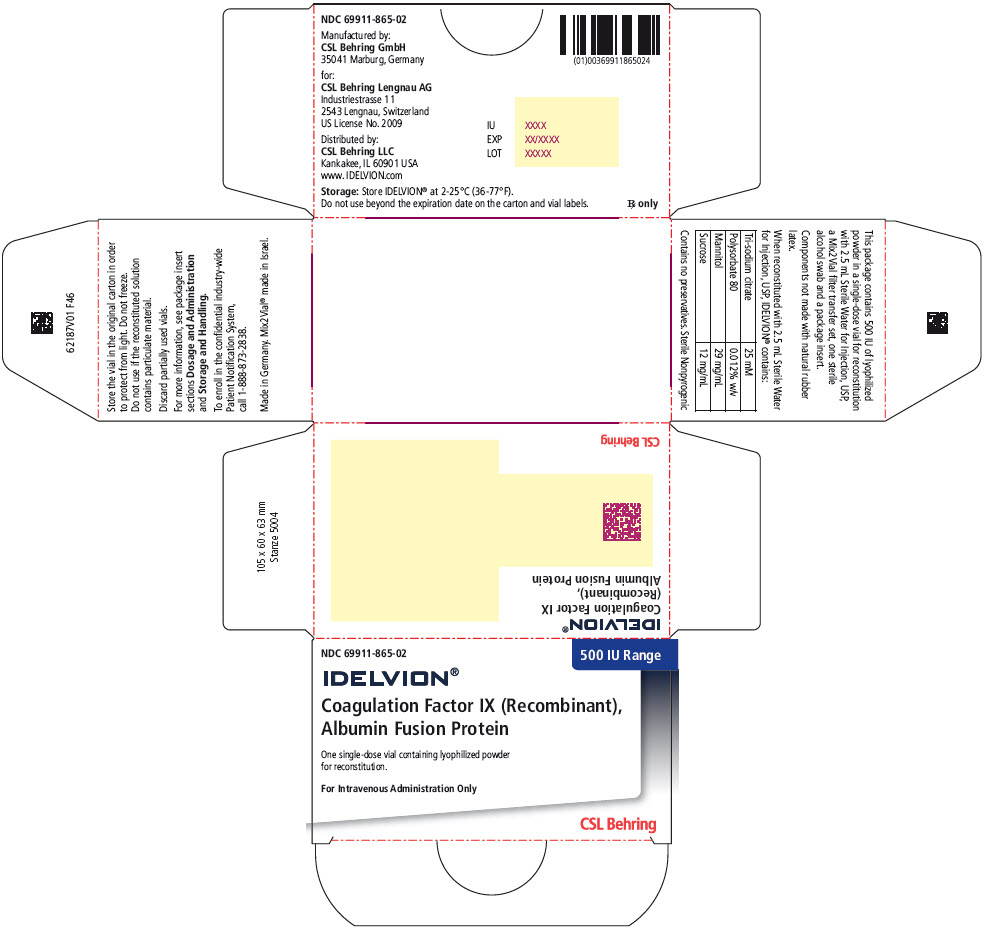
PRINCIPAL DISPLAY PANEL – 500 IU RANGE KIT CARTON
- NDC 69911-865-02
- 500 IU Range
- IDELVION®
- Coagulation Factor IX (Recombinant),
Albumin Fusion Protein - One single-use vial containing lyophilized powder
for reconstitution. - For Intravenous Administration Only
- CSL Behring
SRC: NLM .
