Drizalma Sprinkle
Generic name: duloxetine
Brand names: Cymbalta, Drizalma Sprinkle, Irenka
Drug class: Serotonin-norepinephrine reuptake inhibitors
Medically reviewed by A Ras MD.
What is Drizalma Sprinkle?
Drizalma Sprinkle is prescription medicine used to treat a certain type of depression called Major Depressive Disorder (MDD) in adults, Generalized Anxiety Disorder (GAD) in adults and children 7 to 17 years old, Diabetic Peripheral Neuropathic Pain (DPNP) in adults, Chronic Musculoskeletal Pain in adults.
It is not known if Drizalma Sprinkle is safe and effective for use to treat GAD in children less than 7 years of age.
It is not known if Drizalma Sprinkle is safe and effective for use to treat MDD, DPNP, and Chronic Musculoskeletal Pain in children.
Description
Duloxetine hydrochloride is a selective serotonin and norepinephrine reuptake inhibitor (SNRI). The chemical name of duloxetine hydrochloride is (+)-(S)-N-methyl-γ-(1-naphthyloxy)-2-thiophenepropylamine hydrochloride. The molecular formula is C18H19NOS•HCl, which corresponds to a molecular weight of 333.88. The structural formula is:
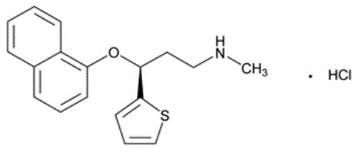
Duloxetine hydrochloride, USP is a white to off-white powder, which is freely soluble in methanol, soluble in dichloromethane, and slightly soluble in water. The molecular formula of duloxetine free base is C18H19NOS and its molecular weight is 297.38.
Each DRIZALMA SPRINKLE (duloxetine delayed-release capsule) for oral administration contains enteric-coated pellets containing a total of 22.4 mg, 33.6 mg, 44.9 mg or 67.3 mg of duloxetine hydrochloride, equivalent to 20 mg, 30 mg, 40 mg or 60 mg of duloxetine free base, respectively. These enteric-coated pellets are designed to prevent degradation of the drug in the acidic environment of the stomach. Inactive ingredients of the pellets include hypromellose, hypromellose phthalate, polyethylene glycol, starch, sucrose, sugar spheres, talc, titanium dioxide, and triethyl citrate. The capsule shell ingredients for 20 mg strength are D&C Yellow 10, FD &C Blue 1, FD &C Red 40, gelatin, sodium lauryl sulfate and titanium dioxide. The capsule shell ingredients for 30 mg strength are FD &C Blue 1, FD &C Red 40 and FD &C Red 3 (present in cap), gelatin, sodium lauryl sulfate and titanium dioxide. The capsule shell ingredients for 40 mg strength are gelatin, sodium lauryl sulfate and titanium dioxide. The capsule shell ingredients for 60 mg strength are D&C Yellow 10 (present in body), FD &C Blue 1, FD &C Red 40, FD &C Red 3 (present in cap), gelatin, sodium lauryl sulfate and titanium dioxide.
The imprinting ink for 20 mg, 30 mg, 40 mg, and 60 mg strength capsules was made of ammonia solution, black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol and shellac.
DRIZALMA SPRINKLE does not comply with the USP dissolution test.
Mechanism of Action
Although the exact mechanisms of the antidepressant, central pain inhibitory and anxiolytic actions of duloxetine in humans are unknown, these actions are believed to be related to its potentiation of serotonergic and noradrenergic activity in the CNS.
What is the most important information I should know about Drizalma Sprinkle?
Drizalma Sprinkle may cause serious side effects, including:
- Increased risk of suicidal thoughts or actions in some children, adolescents, and young adults. Drizalma Sprinkle and other antidepressant medicines may increase suicidal thoughts and actions in some people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts or actions. Some people may have a higher risk of having suicidal thoughts or actions. These include people who have (or have a family history of) depression, bipolar illness (also called manic-depressive illness) or a history of suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions?- Pay close attention to any changes in mood, behavior, actions, thoughts, or feelings, especially sudden changes. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call your healthcare provider or get emergency help right away if you or your family member have any of the following symptoms, especially if they are new, worse, or worry you.
- attempts to commit suicide
- acting on dangerous impulses
- acting aggressive, being angry, or violent
- thoughts about suicide or dying
- new or worse depression
- new or worse anxiety
- panic attacks
- feeling very agitated or restless
- new or worse irritability
- trouble sleeping
- an extreme increase in activity or talking (mania)
- other unusual changes in behavior or mood
Who should not take Drizalma Sprinkle?
Do not take Drizalma Sprinkle if you:
- take a Monoamine Oxidase Inhibitor (MAOI)
- have stopped taking an MAOI in the last 14 days
- are being treated with the antibiotic linezolid or intravenous methylene blue
Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid or intravenous methylene blue.
Do not start taking an MAOI for a least 5 days after you stop treatment with Drizalma Sprinkle.
What should I tell my healthcare provider before taking Drizalma Sprinkle?
Before taking Drizalma Sprinkle, tell your healthcare provider about all your medical conditions, including if you:
- have or have a family history of suicide, bipolar disorder, depression, mania or hypomania
- have liver or kidney problems
- drink alcohol
- have or had had bleeding problems
- have glaucoma (high pressure in the eye)
- have or have had seizures (convulsions)
- have high or low blood pressure
- have diabetes or high blood sugar
- have or have had heart problems or stroke
- have low sodium levels in your blood
- have problems urinating (hesitation) or emptying your bladder (urinary retention)
- are pregnant or plan to become pregnant. Drizalma Sprinkle may harm your unborn baby. Talk to your healthcare provider about the risks to you and your unborn baby if you take Drizalma Sprinkle during pregnancy.
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with Drizalma Sprinkle.
- If you become pregnant during treatment with Drizalma Sprinkle, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants. You can register by calling 1-844-405-6185.
- are breastfeeding or plan to breastfeed. Drizalma Sprinkle passes into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby during treatment with Drizalma Sprinkle.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Drizalma Sprinkle and other medicines may affect each other causing possible serious side effects. Drizalma Sprinkle may affect the way other medicines work and other medicines may affect the way Drizalma Sprinkle works.
Especially tell your healthcare provider if you take:
- other MAOIs
- medicines to treat migraine headaches known as triptans
- tricyclic antidepressants
- fentanyl
- lithium
- tramadol
- tryptophan
- buspirone
- amphetamines
- St. John’s Wort
- other medicines containing desvenlafaxine or venlafaxine
- medicines that can affect blood clotting such as aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), warfarin
Ask your healthcare provider if you are not sure if you are taking any of these medicines. Your healthcare provider can tell you if it is safe to take Drizalma Sprinkle with your other medicines.
Do not start or stop any other medicines during treatment with TREATMENT without talking to your healthcare provider first. Stopping Drizalma Sprinkle suddenly may cause you to have serious side effects. See, “What are the possible side effects of Drizalma Sprinkle?”
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take Drizalma Sprinkle?
- Take Drizalma Sprinkle exactly as your healthcare provider tells you to take it. Do not change your dose or stop taking Drizalma Sprinkle without talking to your healthcare provider.
- Your healthcare provider may need to change the dose of Drizalma Sprinkle until it is the right dose for you.
- Take Drizalma Sprinkle with or without food.
- Swallow Drizalma Sprinkle whole. Do not chew or crush Drizalma Sprinkle.
- If you have trouble swallowing Drizalma Sprinkle whole, you can open the capsule and take the contents with applesauce. See the, Instructions for Use at the end of this Medication Guide for instructions on how to take Drizalma Sprinkle with applesauce.
- See the, Instructions for Use at the end of this Medication Guide for instructions on how to mix and give Drizalma Sprinkle through a nasogastric (NG) tube.
- If you miss a dose of Drizalma Sprinkle, take the missed dose as soon as you remember. If it is almost time for the next dose, skip the missed dose and take your next dose at the regular time. Do not take 2 doses of Drizalma Sprinkle at the same time.
If you take too much Drizalma Sprinkle, call your healthcare provider or poison control center at 1800-222-1222 right away, or go to the nearest hospital emergency room.
What should I avoid while taking Drizalma Sprinkle?
Do not drive, operate heavy machinery, or do other dangerous activities until you know how Drizalma Sprinkle affects you. Drizalma Sprinkle can make you drowsy.
You should not drink large amounts of alcohol during treatment with Drizalma Sprinkle. Drinking large amounts of alcohol during treatment with Drizalma Sprinkle can increase your risk of having serious side effects.
What are the possible side effects of Drizalma Sprinkle?
Drizalma Sprinkle may cause serious side effects, including:
- See “What is the most important information I should know about Drizalma Sprinkle?”
- Liver problems. Drizalma Sprinkle may cause severe liver problems that may cause death. Tell your healthcare provider right away if you develop any of the following symptoms of severe liver problems:
- itching
- right upper abdominal pain
- dark urine
- yellow skin or eyes
- enlarged liver
- increased liver enzymes
- Decreased in blood pressure (orthostatic hypotension). You may feel lightheaded or faint when you rise too quickly from a sitting or lying position, especially when you start or restart treatment or when the dose is changed.
- Falls and fainting. Drizalma Sprinkle may cause you to feel sleepy or dizzy, may cause a decrease in your blood pressure when you rise to quickly from a sitting or lying position, and can slow your thinking and motor skills which may lead to falls that have caused fractures or other serious injuries.
- Serotonin syndrome. A potentially life-threatening problem called serotonin syndrome can happen when you take Drizalma Sprinkle with certain other medicines. See, “Who should not take Drizalma Sprinkle?” Call your healthcare provider or go to the nearest hospital emergency room right away if you have any of the following signs and symptoms of serotonin syndrome:
- agitation
- seeing or hearing things that are not real (hallucinations)
- confusion
- coma
- fast heart beat
- blood pressure changes
- dizziness
- sweating
- flushing
- high body temperature (hyperthermia)
- tremors, stiff muscles, or muscle twitching
- loss of coordination
- seizures
- nausea, vomiting, diarrhea
- Abnormal bleeding. Taking Drizalma Sprinkle with aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), or blood thinners may add to this risk. Tell your healthcare provider right away about any unusual bleeding or bruising.
- Severe skin reactions. Drizalma Sprinkle may cause serious skin reactions that may need to be treated in a hospital and may be life-threating. Stop taking Drizalma Sprinkle and call your healthcare provider or get emergency help right away if you develop skin blisters, peeling rash, sores in the mouth, hives or any other allergic reactions during treatment with Drizalma Sprinkle.
- Discontinuation syndrome. Suddenly stopping Drizalma Sprinkle when you take higher doses may cause you to have serious side effects. Your healthcare provider may want to decrease your dose slowly. Symptoms may include:
- dizziness
- nausea
- headache
- irritability and agitation
- problems sleeping
- diarrhea
- anxiety
- tiredness
- abnormal dreams
- sweating
- confusion
- changes in your mood
- seizures
- electric shock sensation (paresthesia)
- hypomania
- ringing in your ears (tinnitus)
- Manic episodes. Manic episodes may happen in people with bipolar disorder who take Drizalma Sprinkle. Symptoms may include:
- greatly increased energy
- severe trouble sleeping
- racing thoughts
- reckless behavior
- unusually grand ideas
- excessive happiness or irritability
- talking more or faster than usual
- Eye problems (angle-closure glaucoma). Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are.
- Seizures (convulsions).
- Increases in blood pressure. Your healthcare provider should check your blood pressure regularly during treatment with Drizalma Sprinkle. If you have high blood pressure, it should be controlled before you start treatment with Drizalma Sprinkle.
- Low sodium levels in your blood (hyponatremia). Low sodium levels can happen during treatment with Drizalma Sprinkle. Low sodium levels in your blood may be serious and may cause death. Elderly people may be at greater risk for this. Signs and Symptoms of low sodium levels in your blood may include: In severe or more sudden cases, signs and symptoms include:
- headache
- difficulty concentrating
- memory changes
- confusion
- weakness and unsteadiness on your feet which can lead to falls
- hallucinations (seeing or hearing things that are not real)
- fainting
- seizures
- coma
- respiratory arrest
- death
- Problems with urination. Drizalma Sprinkle may cause you to have problems with urination including decreased urine flow and being unable to pass any urine. Tell your healthcare provider if you develop any problems with urine flow during treatment with Drizalma Sprinkle.
- Your healthcare provider may tell you to stop taking Drizalma Sprinkle if you develop serious side effects during treatment with Drizalma Sprinkle.
The most common side effects of Drizalma Sprinkle, include:
- nausea
- dry mouth
- sleepiness
- constipation
- fatigue
- loss of appetite
- increased sweating
Height and weight changes in children and adolescents may happen during treatment with Drizalma Sprinkle. Your healthcare provider should check your child’s or adolescent’s height and weight during treatment with Drizalma Sprinkle.
These are not all the possible side effects of Drizalma Sprinkle.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Drizalma Sprinkle
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Drizalma Sprinkle for a condition for which it was not prescribed. Do not give Drizalma Sprinkle to other people, even if they have the same symptoms that you have. It may harm them. You may ask your healthcare provider or pharmacist for information about Drizalma Sprinkle that is written for healthcare professionals.
How should I store Drizalma Sprinkle?
- Store Drizalma Sprinkle at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Drizalma Sprinkle in a tightly closed container.
Keep Drizalma Sprinkle and all medicines out of the reach of children.
What are the ingredients in Drizalma Sprinkle?
Active ingredient: duloxetine hydrochloride
Inactive ingredients: hypromellose, hypromellose phthalate, polyethylene glycol, starch, sucrose, sugar spheres, talc, titanium dioxide, and triethyl citrate.
- The capsule shell ingredients for 20 mg strength are D&C Yellow 10, FD&C Blue 1, FD&C Red 40, gelatin, sodium lauryl sulfate and titanium dioxide.
- The capsule shell ingredients for 30 mg strength are FD&C Blue 1, FD&C Red 40 and FD&C Red 3 (present in cap), gelatin, sodium lauryl sulfate and titanium dioxide.
- The capsule shell ingredients for 40 mg strength are gelatin, sodium lauryl sulfate and titanium dioxide.
- The capsule shell ingredients for 60 mg strength are D&C Yellow 10 (present in body), FD&C Blue 1, FD&C Red 40, FD&C Red 3 (present in cap), gelatin, sodium lauryl sulfate and titanium dioxide.
- The imprinting ink for 20 mg, 30 mg, 40 mg, and 60 mg strength capsules was made of ammonia solution, black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol and shellac.
For more information call Sun Pharmaceutical Industries, Inc. at 1-800-818-4555.
Instructions for use for Drizalma Sprinke
DrizalLabelma Sprinkle (dri zal’ mah)
(duloxetine delayed-release capsules)
Taking Drizalma Sprinkle with applesauce:
- Carefully open the Drizalma Sprinkle capsule.
- Sprinkle the pellets from the capsules on 1 tablespoonful of applesauce.
- Swallow the applesauce and pellets mixture right away. Do not chew the pellets.
- Do not save the applesauce and pellets mixture for later use. Throw away any remaining applesauce and pellets mixture.
Giving Drizalma Sprinkle through a nasogastric tube (NG tube) 12 French or larger as prescribed by your healthcare provider:
For people who have a NG tube in place, Drizalma Sprinkle may be given as follows:
- 1. Remove the plunger from a 60 mL catheter tipped syringe.
- 2. Carefully open the Drizalma Sprinkle capsule and empty the pellets into the catheter tipped syringe barrel.
- 3. Add 50 mL of water to the pellets that are inside of the catheter tipped syringe barrel. Do not use other liquids.
- 4. Replace the plunger and gently shake the syringe well for approximately 10 seconds.
- 5. Insert the catheter tipped syringe to a NG tube (≥12 French).
- 6. Give the mixture right away through the NG tube into the stomach. Do not save the mixture for later use.
- 7. After giving the mixture, the NG tube should be flushed with 15 mL of additional water.
How should I store Drizalma Sprinkle?
- Store Drizalma Sprinkle at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Drizalma Sprinkle in a tightly closed container.
Keep Drizalma Sprinkle and all medicines out of the reach of children.
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 47335-616-30
- Drizalma Sprinkle™
- (duloxetine delayed-release capsules)
- 20 mg
- 30 Capsules
- Rx only SUN PHARMA

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 47335-617-30
- Drizalma Sprinkle™
- (duloxetine delayed-release capsules)
- 30 mg
- 90 Capsules
- Rx only SUN PHARMA

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 47335-618-30
- Drizalma Sprinkle™
- (duloxetine delayed-release capsules)
- 40 mg
- 30 Capsules
- Rx only SUN PHARMA

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 47335-619-30
- Drizalma Sprinkle™
- (duloxetine delayed-release capsules)
- 60 mg
- 30 Capsules
- Rx only SUN PHARMA

SRC: NLM .
