Cinqair
Generic name: reslizumab
Drug class: Interleukin inhibitors
Medically reviewed by A Ras MD.
What is Cinqair?
Cinqair is a prescription medicine used with other asthma medicines for the maintenance treatment of asthma in people aged 18 years of age and older whose asthma is not controlled with the current asthma medicines. When added to other medicines for asthma, Cinqair helps prevent severe asthma attacks (exacerbations) and can improve your breathing. Medicines such as Cinqair reduce blood eosinophils. Eosinophils are a type of white blood cell that may contribute to your asthma.
Cinqair is not used to treat other problems caused by eosinophils. Cinqair is not used to treat sudden breathing problems.
It is not known if Cinqair is safe and effective in children less than 18 years of age.
Description
CINQAIR (reslizumab) is a humanized interleukin-5 antagonist monoclonal antibody (IgG4κ). Reslizumab is produced by recombinant DNA technology in murine myeloma non-secreting 0 (NS0) cells. Reslizumab has a molecular weight of approximately 147 kDa.
CINQAIR is a sterile, preservative-free, clear to slightly hazy/opalescent, colorless to slightly yellow solution (injection) for intravenous infusion. Since CINQAIR is a protein, proteinaceous particles may be present in the solution that appear as translucent to white, amorphous particulates. Each single-use vial contains 100 mg reslizumab in 10 mL. Each mL contains 10 mg of reslizumab, glacial acetic acid (0.12 mg), sodium acetate trihydrate (2.45 mg), and sucrose (70 mg), with a pH of 5.5.
Mechanism of Action
Reslizumab is an interleukin-5 antagonist (IgG4, kappa). IL-5 is the major cytokine responsible for the growth and differentiation, recruitment, activation, and survival of eosinophils. Reslizumab binds to IL-5 with a dissociation constant of 81 pM, inhibiting the bioactivity of IL-5 by blocking its binding to the alpha chain of the IL-5 receptor complex expressed on the eosinophil surface. Inflammation is an important component in the pathogenesis of asthma. Multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) are involved in inflammation. Reslizumab, by inhibiting IL-5 signaling, reduces the production and survival of eosinophils; however, the mechanism of reslizumab action in asthma has not been definitively established.
What is the most important information I should know about Cinqair?
Cinqair can cause serious side effects, including:
- Serious allergic reactions (anaphylaxis). Serious allergic reactions can happen right after you receive your Cinqair infusion. These reactions can cause death. Allergic reactions sometimes do not happen right away. Your healthcare provider will watch you during and after you receive your Cinqair infusion for any signs of a reaction. Tell your healthcare provider right away if you have any of the following symptoms that may be associated with an allergic reaction:
- breathing problems
- flushing
- itching
- symptoms of low blood pressure (fainting, dizziness, light headedness, confusion, fast heart beat)
- paleness
- skin rash (hives)
- swelling of your face, lips, mouth, or tongue
- nausea
- abdominal discomfort
Who should not use Cinqair?
Do not receive Cinqair if you are allergic to reslizumab or any of the ingredients in Cinqair. See the end of this leaflet for a complete list of ingredients in Cinqair.
What should I tell my healthcare provider before using Cinqair?
Before receiving Cinqair, tell your healthcare provider about all of your medical conditions, including if you:
- are taking oral or inhaled corticosteroid medicines. Do not stop taking your corticosteroid unless your healthcare provider tells you to stop. This may cause other symptoms that were controlled by the corticosteroid medicine to come back.
- have or have had cancer (malignancy).
- have a parasitic (helminth) infection.
- are pregnant or plan to become pregnant. It is not known if Cinqair will harm your unborn baby. Tell your healthcare provider if you become pregnant during your treatment with Cinqair.
- are breastfeeding or plan to breastfeed. It is not known if Cinqair passes into your breast milk. You and your healthcare provider should decide if you will receive Cinqair and breastfeed. Talk to your healthcare provider about the best way to feed your baby if you receive Cinqair.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Do not stop taking your other asthma medicines unless your healthcare provider tells you to.
How should I use Cinqair?
You will be given Cinqair by a healthcare provider using a needle placed in a vein (intravenous infusion) 1 time every 4 weeks. It will take about 20 to 50 minutes to receive the full dose of Cinqair.
What are the possible side effects of Cinqair?
Cinqair may cause serious side effects, including:
- See “What is the most important information I should know about Cinqair?”
- abnormal growth of cells or tissue in your body that may or may not be cancer (malignancy)
The most common side effects of Cinqair include throat pain.
These are not all the possible side effects of Cinqair.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Cinqair
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Cinqair for a condition for which it was not prescribed. You can ask your pharmacist or healthcare provider for information about Cinqair that is written for healthcare professionals.
How should I store Cinqair?
Refrigerate at 2 ºC to 8ºC (36°F to 46°F). Do not freeze. Do not shake. Protect the vials from light by storing in the original package until time of use.
What are the ingredients in Cinqair?
Active ingredient: reslizumab
Inactive ingredients: sodium acetate, acetic acid, sucrose
Label
PACKAGE/LABEL DISPLAY PANEL NDC 59310-610-31
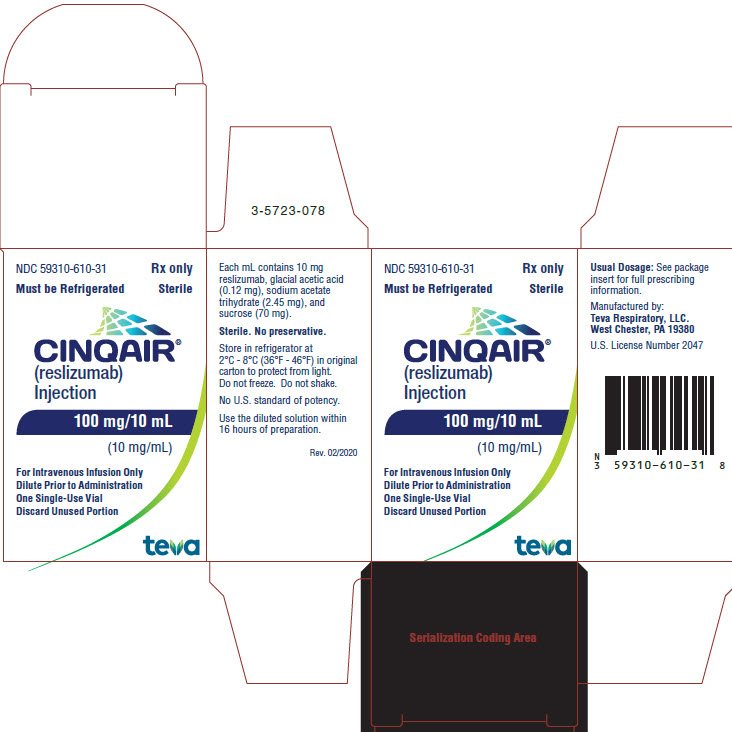
- NDC 59310-610-31
- Rx only
- Must be Refrigerated Sterile
- CINQAIR® (reslizumab) Injection 100 mg/10 mL (10 mg/mL)
- For Intravenous Infusion Only
- Dilute Prior to Administration
- One Single-Use Vial
- Discard Unused Portion
TEVA
PACKAGE/LABEL DISPLAY PANEL NDC 59310-610-33
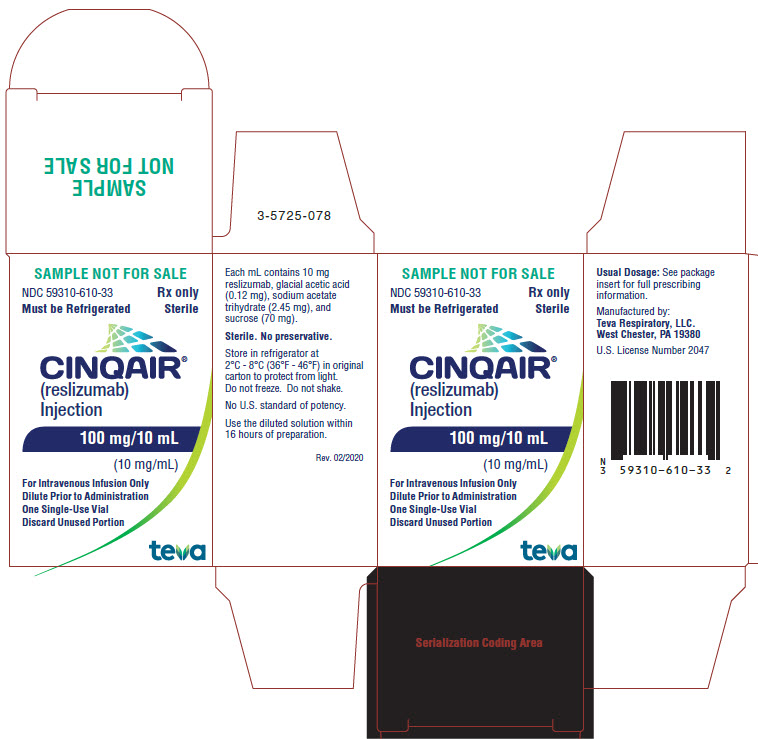
- NDC 59310-610-33
- Rx only
- Must be Refrigerated
- Sterile
- CINQAIR® (reslizumab) Injection 100 mg/10 mL (10 mg/mL)
- For Intravenous Infusion Only
- Dilute Prior to Administration
- One Single-Use Vial
- Discard Unused Portion
- TEVA
SRC: NLM .
