Cellcept
Generic name: mycophenolate mofetil (oral/injection)
Drug class: Selective immunosuppressants
Medically reviewed by A Ras MD.
What is Cellcept?
Cellcept is a prescription medicine to prevent rejection (antirejection medicine) in people who have received a kidney, heart or liver transplant. Rejection is when the body’s immune system perceives the new organ as a “foreign” threat and attacks it. Cellcept is used with other medicines containing cyclosporine and corticosteroids.
Description
CellCept (mycophenolate mofetil) is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor.
The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate. It has an empirical formula of C23H31NO7, a molecular weight of 433.50, and the following structural formula:
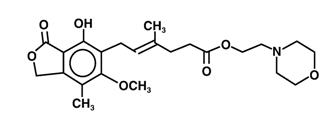
Mycophenolate mofetil is a white to off-white crystalline powder. It is slightly soluble in water (43 µg/mL at pH 7.4); the solubility increases in acidic medium (4.27 mg/mL at pH 3.6). It is freely soluble in acetone, soluble in methanol, and sparingly soluble in ethanol. The apparent partition coefficient in 1-octanol/water (pH 7.4) buffer solution is 238. The pKa values for mycophenolate mofetil are 5.6 for the morpholino group and 8.5 for the phenolic group.
Mycophenolate mofetil hydrochloride has a solubility of 65.8 mg/mL in 5% Dextrose Injection USP (D5W). The pH of the reconstituted solution is 2.4 to 4.1.
CellCept is available for oral administration as capsules containing 250 mg of mycophenolate mofetil, tablets containing 500 mg of mycophenolate mofetil, and as a powder for oral suspension, which when constituted contains 200 mg/mL mycophenolate mofetil.
Inactive ingredients in CellCept 250 mg capsules include croscarmellose sodium, magnesium stearate, povidone (K-90) and pregelatinized starch. The capsule shells contain black iron oxide, FD&C blue #2, gelatin, red iron oxide, silicon dioxide, sodium lauryl sulfate, titanium dioxide, and yellow iron oxide.
Inactive ingredients in CellCept 500 mg tablets include black iron oxide, croscarmellose sodium, FD&C blue #2 aluminum lake, hydroxypropyl cellulose, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, povidone (K-90), red iron oxide, talc, and titanium dioxide; may also contain ammonium hydroxide, ethyl alcohol, methyl alcohol, n-butyl alcohol, propylene glycol, and shellac.
Inactive ingredients in CellCept Oral Suspension include aspartame, citric acid anhydrous, colloidal silicon dioxide, methylparaben, mixed fruit flavor, sodium citrate dihydrate, sorbitol, soybean lecithin, and xanthan gum.
CellCept Intravenous is the hydrochloride salt of mycophenolate mofetil. The chemical name for the hydrochloride salt of mycophenolate mofetil is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate hydrochloride. It has an empirical formula of C23H31NO7 HCl and a molecular weight of 469.96.
CellCept Intravenous is available as a sterile white to off-white lyophilized powder in vials containing mycophenolate mofetil hydrochloride for administration by intravenous infusion only. Each vial of CellCept Intravenous contains the equivalent of 500 mg mycophenolate mofetil as the hydrochloride salt. The inactive ingredients are polysorbate 80, 25 mg, and citric acid, 5 mg. Sodium hydroxide may have been used in the manufacture of CellCept Intravenous to adjust the pH. Reconstitution and dilution with 5% Dextrose Injection USP yields a slightly yellow solution of mycophenolate mofetil, 6 mg/mL.
Mechanism of Action
Mycophenolate mofetil has been demonstrated in experimental animal models to prolong the survival of allogeneic transplants (kidney, heart, liver, intestine, limb, small bowel, pancreatic islets, and bone marrow).
Mycophenolate mofetil has also been shown to reverse ongoing acute rejection in the canine renal and rat cardiac allograft models. Mycophenolate mofetil also inhibited proliferative arteriopathy in experimental models of aortic and cardiac allografts in rats, as well as in primate cardiac xenografts. Mycophenolate mofetil was used alone or in combination with other immunosuppressive agents in these studies. Mycophenolate mofetil has been demonstrated to inhibit immunologically mediated inflammatory responses in animal models and to inhibit tumor development and prolong survival in murine tumor transplant models.
Mycophenolate mofetil is rapidly absorbed following oral administration and hydrolyzed to form MPA, which is the active metabolite. MPA is a potent, selective, uncompetitive, and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), and therefore inhibits the de novo pathway of guanosine nucleotide synthesis without incorporation into DNA. Because T- and B-lymphocytes are critically dependent for their proliferation on de novo synthesis of purines, whereas other cell types can utilize salvage pathways, MPA has potent cytostatic effects on lymphocytes. MPA inhibits proliferative responses of T- and B-lymphocytes to both mitogenic and allospecific stimulation. Addition of guanosine or deoxyguanosine reverses the cytostatic effects of MPA on lymphocytes. MPA also suppresses antibody formation by B-lymphocytes. MPA prevents the glycosylation of lymphocyte and monocyte glycoproteins that are involved in intercellular adhesion to endothelial cells and may inhibit recruitment of leukocytes into sites of inflammation and graft rejection. Mycophenolate mofetil did not inhibit early events in the activation of human peripheral blood mononuclear cells, such as the production of interleukin-1 (IL-1) and interleukin-2 (IL-2), but did block the coupling of these events to DNA synthesis and proliferation.
What is the most important information I should know about Cellcept?
Cellcept can cause serious side effects, including:
1. Increased risk of loss of a pregnancy (miscarriage) and higher risk of birth defects. Females who take Cellcept during pregnancy have a higher risk of miscarriage during the first 3 months (first trimester), and a higher risk that their baby will be born with birth defects.
- If you are a female who can become pregnant, your doctor must talk with you about acceptable birth control methods (contraceptive counseling) to use while taking Cellcept. You should have 1 pregnancy test immediately before starting Cellcept and another pregnancy test 8 to 10 days later. Pregnancy tests should be repeated during routine follow-up visits with your doctor. Talk to your doctor about the results of all of your pregnancy tests.
You must use acceptable birth control during your entire Cellcept treatment and for 6 weeks after stopping Cellcept, unless at any time you choose to avoid sexual intercourse (abstinence) with a man completely. Cellcept decreases blood levels of the hormones in birth control pills that you take by mouth. Birth control pills may not work as well while you take Cellcept, and you could become pregnant. If you take birth control pills while using Cellcept you must also use another form of birth control. Talk to your doctor about other birth control methods that you can use while taking Cellcept. - If you are a sexually active male whose female partner can become pregnant while you are taking Cellcept, use effective contraception during treatment and for at least 90 days after stopping Cellcept.
- If you plan to become pregnant, talk with your doctor. Your doctor will decide if other medicines to prevent rejection may be right for you.
- If you become pregnant while taking Cellcept, do not stop taking Cellcept. Call your doctor right away. You and your doctor may decide that other medicines to prevent rejection may be right for you. You and your doctor should report your pregnancy to the Mycophenolate Pregnancy Registry.
- By phone at 1-800-617-8191 or
- By visiting the REMS website at: www.mycophenolateREMS.comThe purpose of this registry is to gather information about the health of you and your baby. You can report you pregnancy to the registry either:
2. Increased risk of getting certain cancers. People who take Cellcept have a higher risk of getting lymphoma, and other cancers, especially skin cancer. Tell your doctor if you have:
- unexplained fever, prolonged tiredness, weight loss or lymph node swelling
- a brown or black skin lesion with uneven borders, or one part of the lesion does not look like the other
- a change in the size and color of a mole
- a new skin lesion or bump
- any other changes to your health
3. Increased risk of getting serious infections. Cellcept weakens the body’s immune system and affects your ability to fight infections. Serious infections can happen with Cellcept and can lead to hospitalizations and death. These serious infections can include:
- Viral infections. Certain viruses can live in your body and cause active infections when your immune system is weak. Viral infections that can happen with Cellcept include:
- Shingles, other herpes infections, and cytomegalovirus (CMV). CMV can cause serious tissue and blood infections.
- BK virus. BK virus can affect how your kidney works and cause your transplanted kidney to fail.
- Hepatitis B and C viruses. Hepatitis viruses can affect how your liver works. Talk to your doctor about how hepatitis viruses may affect you.
- A brain infection called Progressive Multifocal Leukoencephalopathy (PML). In some patients, Cellcept may cause an infection of the brain that may cause death. You are at risk for this brain infection because you have a weakened immune system. Call your doctor right away if you have any of the following symptoms:
- weakness on one side of the body
- you do not care about things you usually care about (apathy)
- you are confused or have problems thinking
- you cannot control your muscles
- Fungal infections. Yeasts and other types of fungal infections can happen with Cellcept and can cause serious tissue and blood infections (See “What are the possible side effects of Cellcept?”).Call your doctor right away if you have any of the following signs and symptoms of infection:
- temperature of 100.5°F or greater
- cold symptoms, such as a runny nose or sore throat
- flu symptoms, such as an upset stomach, stomach pain, vomiting or diarrhea
- earache or headache
- pain during urination
- white patches in the mouth or throat
- unexpected bruising or bleeding
- cuts, scrapes or incisions that are red, warm and oozing pus
See “What are the possible side effects of Cellcept?” below for information about other serious side effects.
Who should not take Cellcept?
Do not take Cellcept if you are allergic to mycophenolate mofetil or any of the ingredients in Cellcept. See the end of this Medication Guide for a complete list of ingredients in Cellcept.
What should I tell my healthcare provider before taking Cellcept?
Tell your doctor about all of your medical conditions, including if you:
- have any digestive problems, such as ulcers.
- have Phenylketonuria (PKU). Cellcept oral suspension contains aspartame (a source of phenylalanine).
- have Lesch-Nyhan syndrome, Kelley-Seegmiller syndrome, or another rare inherited deficiency hypoxanthine-guanine phosphoribosyl-transferase (HGPRT). You should not take Cellcept if you have one of these disorders.
- plan to receive any vaccines. People taking Cellcept should not receive live vaccines. Some vaccines may not work as well during treatment with Cellcept.
- are pregnant or plan to become pregnant. See “What is the most important information I should know about Cellcept?”
- are breastfeeding or plan to breastfeed. It is not known if Cellcept passes into breast milk. You and your doctor will decide if you will take Cellcept or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Some medicines may affect the way Cellcept works, and Cellcept may affect how some medicines work.
Especially tell your doctor if you take:
- birth control pills (oral contraceptives). See “What is the most important information I should know about Cellcept?”
- sevelamer (Renagel, Renvela). These products should be taken at least 2 hours after taking Cellcept.
- acyclovir (Zovirax), valacyclovir (Valtrex), ganciclovir (cytovene-iv, Vitrasert), valganciclovir (Valcyte).
- rifampin (Rifater, Rifamate, Rimactane, Rifadin).
- antacids that contain magnesium and aluminum (Cellcept and the antacid should not be taken at the same time).
- proton pump inhibitors (PPIs) (Prevacid, Protonix).
- sulfamethoxazole/trimethoprim (Bactrim, Bactrim ds).
- norfloxacin (Noroxin) and metronidazole (Flagyl, Flagyl ER, Flagyl IV, Metro IV, Helidac, Pylera).
- ciprofloxacin (Cipro, Cipro XR, Ciloxan, Proquin XR) and amoxicillin plus clavulanic acid (Augmentin, Augmentin XR).
- azathioprine (Azasan, Imuran).
- cholestyramine (Questran Light, Questran, Locholest Light, Locholest, Prevalite).
Know the medicines you take. Keep a list of them to show to your doctor or nurse and pharmacist when you get a new medicine. Do not take any new medicine without talking with your doctor.
How should I take Cellcept?
- Take Cellcept exactly as prescribed.
- Do not stop taking Cellcept or change the dose unless your doctor tells you to.
- If you miss a dose of Cellcept, or you are not sure when you took your last dose, take your prescribed dose of Cellcept as soon as you remember. If your next dose is less than 2 hours away, skip the missed dose and take your next dose at your normal scheduled time. Do not take 2 doses at the same time. Call your doctor if you are not sure what to do.
- Take Cellcept capsules, tablets and oral suspension on an empty stomach, unless your doctor tells you otherwise. Do not crush Cellcept tablets.
- Do not open or crush Cellcept capsules.
- If you are not able to swallow Cellcept tablets or capsules, your doctor may prescribe Cellcept Oral Suspension. This is a liquid form of Cellcept. Your pharmacist will mix the medicine before you pick it up from a pharmacy.
- Do not mix Cellcept Oral Suspension with any other medicine. Cellcept Oral Suspension should not be mixed with any type of liquids before taking the dose. See the Instructions for Use with your medication for detailed instructions about how to take Cellcept Oral Suspension the right way.
- Do not breathe in (inhale) or let Cellcept powder or oral suspension come in contact with your skin or mucous membranes.
- If you accidentally get the powder or oral suspension on the skin, wash the area well with soap and water.
- If you accidentally get the powder or oral suspension in your eyes or other mucous membranes, flush with plain water.
- If you take too much Cellcept, call your doctor or the poison control center right away.
What should I avoid while taking Cellcept?
- Avoid becoming pregnant. See “What is the most important information I should know about Cellcept?”
- Limit the amount of time you spend in sunlight. Avoid using tanning beds or sunlamps. People who take Cellcept have a higher risk of getting skin cancer (See “What is the most important information I should know about Cellcept?”). Wear protective clothing when you are in the sun and use a broad-spectrum sunscreen with a high protection factor. This is especially important if your skin is very fair or if you have a family history of skin cancer.
- You should not donate blood while taking Cellcept and for at least 6 weeks after stopping Cellcept.
- You should not donate sperm while taking Cellcept and for 90 days after stopping Cellcept.
- Cellcept may influence your ability to drive and use machines (See “What are the possible side effects of Cellcept?”) If you experience drowsiness, confusion, dizziness, tremor, or low blood pressure during treatment with Cellcept, you should be cautious about driving or using heavy machines.
What are the possible side effects of Cellcept?
Cellcept can cause serious side effects, including:
- See “What is the most important information I should know about Cellcept?”
- Low blood cell counts. People taking high doses of Cellcept each day may have a decrease in blood counts, including:
- white blood cells, especially neutrophils. Neutrophils fight against bacterial infections. You have a higher chance of getting an infection when your white blood cell count is low. This is most common from 1 month to 6 months after your transplant.
- red blood cells. Red blood cells carry oxygen to your body tissues. You have a higher chance of getting severe anemia when your red blood cell count is low.
- platelets. Platelets help with blood clotting.Your doctor will do blood tests before you start taking Cellcept and during treatment with Cellcept to check your blood cell counts. Tell your doctor right away if you have any signs of infection (See “What is the most important information I should know about Cellcept?”), including any unexpected bruising or bleeding. Also, tell your doctor if you have unusual tiredness, lack of energy, dizziness or fainting.
- Stomach problems. Stomach problems including intestinal bleeding, a tear in your intestinal wall (perforation) or stomach ulcers can happen in people who take Cellcept. Bleeding can be severe and you may have to be hospitalized for treatment. Call your doctor right away if you have sudden or severe stomach-area pain or stomach-area pain that does not go away, or if you have diarrhea.
The most common side effects of Cellcept include:
- diarrhea
- blood problems including low white and red blood cell counts
- infections
- blood pressure problems
- fast heart beat
- swelling of the lower legs, ankles and feet
- changes in laboratory blood levels, including high levels of blood sugar (hyperglycemia)
- stomach problems including diarrhea, constipation, nausea and vomiting
- rash
- nervous system problems such as headache, dizziness and tremor
Side effects that can happen more often in children than in adults taking Cellcept include:
- stomach area pain
- fever
- infection
- pain
- blood infection (sepsis)
- diarrhea
- vomiting
- sore throat
- colds (respiratory tract infections)
- high blood pressure
- low white blood cell count
- low red blood cell count
These are not all of the possible side effects of Cellcept. Tell your doctor about any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Genentech at 1-888-835-2555.
General Information about the safe and effective use of Cellcept
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Cellcept for a condition for which it was not prescribed. Do not give Cellcept to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Cellcept. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Cellcept that is written for health professionals.
How should I store Cellcept?
- Store Cellcept capsules and tablets at room temperature between 59°F to 86°F (15°C to 30°C).
- Keep Cellcept tablets in the light resistant container that it comes in.
- Store Cellcept Oral Suspension at room temperature between 59°F to 86°F (15°C to 30°C), for up to 60 days. You can also store Cellcept Oral Suspension in the refrigerator between 36°F to 46°F (2°C to 8°C). Do not freeze.
- Store Cellcept powder and reconstituted infusion solution at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Keep Cellcept and all medicines out of the reach of children.
What are the ingredients in Cellcept?
Active Ingredient: mycophenolate mofetil
Inactive Ingredients:
Capsules: croscarmellose sodium, magnesium stearate, povidone (K-90) and pregelatinized starch. The capsule shells contain black iron oxide, FD&C blue #2, gelatin, red iron oxide, silicon dioxide, sodium lauryl sulfate, titanium dioxide, and yellow iron oxide.
Tablets: black iron oxide, croscarmellose sodium, FD&C blue #2 aluminum lake, hydroxypropyl cellulose, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, povidone (K-90), red iron oxide, talc, and titanium dioxide; may also contain ammonium hydroxide, ethyl alcohol, methyl alcohol, n-butyl alcohol, propylene glycol, and shellac.
Oral suspension: aspartame, citric acid anhydrous, colloidal silicon dioxide, methylparaben, mixed fruit flavor, sodium citrate dihydrate, sorbitol, soybean lecithin, and xanthan gum.
Intravenous: polysorbate 80, and citric acid. Sodium hydroxide and hydrochloric acid may have been used in the manufacture of Cellcept Intravenous to adjust the pH.
For more information, call 1-888-835-2555 or visit www.gene.com/patients/medicines/cellcept.
Instructions for use for Cellcept Oral Suspension
Cellcept [Sel-Sept]
Mycophenolate mofetile for oral suspension
Important:
- Always use the oral dispenser provided with Cellcept Oral Suspension to make sure you measure the right amount of medicine.
- Call your pharmacist if your oral dispenser is lost or damaged.
- Your pharmacist will write the expiration date on your Cellcept Oral Suspension bottle label. Do not use after the expiration date.
- Ask your doctor or pharmacist if you have any questions or are unsure about how to take your dose of medicine.
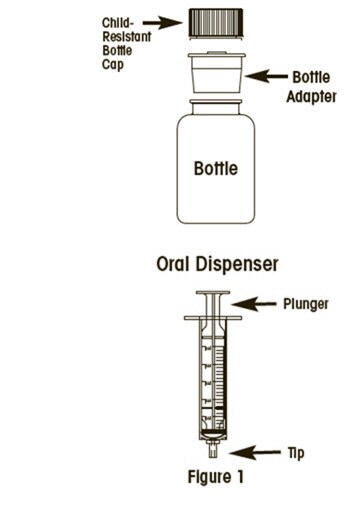
To take a dose of Cellcept Oral Suspension, you will need the bottle of medicine and an oral dispenser provided with the medicine (See Figure 1). Your pharmacist will insert the bottle adapter in the Cellcept Oral Suspension bottle.
Step 1: With the child-resistant cap on the bottle, shake the bottle well for about 5 seconds before each use.
Step 2: Open the bottle by pressing down on the child-resistant bottle cap and turning it counter-clockwise (to the left). Do not throw away the child-resistant bottle cap.
Step 3: Before inserting the tip of the oral dispenser into the bottle adapter, push the plunger completely down toward the tip of the oral dispenser. Insert the tip firmly into the opening of the bottle adapter.
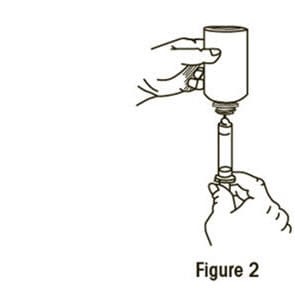
Step 4: Carefully turn the bottle upside down with the oral dispenser in place. Slowly pull the plunger down to withdraw your prescribed dose. Do not pull the plunger out of the oral dispenser (See Figure 2).
Step 5: Leave the oral dispenser in the bottle and turn the bottle to an upright position. Slowly remove the oral dispenser from the bottle.
Step 6: Place the tip of the oral dispenser in the patient’s mouth and slowly push the plunger down until the oral dispenser is empty. The Cellcept oral suspension that is in the oral dispenser should not be mixed with any type of liquids before taking the dose.
Step 7: Put the child-resistant bottle cap back on the bottle after each use.
Step 8: Rinse the oral dispenser under running tap water after each use:
- Remove the plunger from the oral dispenser.
- Rinse the oral dispenser and plunger with water and let them air dry.
- When the oral dispenser and plunger are dry, put the plunger back in the oral dispenser for the next use.
Important:
- Do not let Cellcept Oral Suspension come in contact with the skin. If this happens, wash the skin well with soap and water.
- If you spill any oral suspension, wipe it up using paper towels wet with water. Put the child-resistant bottle cap back on the bottle and wipe the outside of the bottle with wet paper towels.
How should I store Cellcept Oral Suspension?
- Store the Cellcept Oral Suspension at room temperature between 59°F to 86°F (15°C to 30°C), for up to 60 days. You can also store Cellcept Oral Suspension in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze.
Keep Cellcept Oral Suspension and all medicines out of the reach of children.
Instructions for use last revised 08/2018.
Label
PRINCIPAL DISPLAY PANEL – 250 MG CAPSULE CARTON
- NDC 21695-171-00
- CellCept®
(mycophenolate
mofetil capsules) - 250 mg
- Each capsule contains
250 mg mycophenolate mofetil. - Rx only
- Attention Pharmacist: Dispense the
accompanying Medication Guide to each
patient. For additional Medication Guides
call 1-800-617-8191 or visit
www.gene.com/gene/
products/information/cellcept. - 100 capsules
- Rebel Distributors Corp
SRC: NLM .
