Zytiga
Generic name: abiraterone
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Zytiga?
Zytiga is a prescription medicine that is used along with prednisone. Zytiga is used to treat men with prostate cancer that has spread to other parts of the body.
It is not known if Zytiga is safe and effective in females or children.
Description
Abiraterone acetate, the active ingredient of ZYTIGA is the acetyl ester of abiraterone. Abiraterone is an inhibitor of CYP17 (17α-hydroxylase/C17,20-lyase). Each ZYTIGA tablet contains either 250 mg or 500 mg of abiraterone acetate. Abiraterone acetate is designated chemically as (3β)-17-(3-pyridinyl) androsta-5,16-dien-3-yl acetate and its structure is:
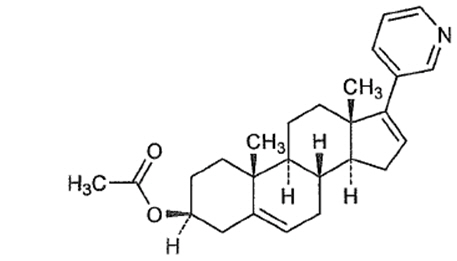
Abiraterone acetate is a white to off-white, non-hygroscopic, crystalline powder. Its molecular formula is C26H33NO2 and it has a molecular weight of 391.55. Abiraterone acetate is a lipophilic compound with an octanol-water partition coefficient of 5.12 (Log P) and is practically insoluble in water. The pKa of the aromatic nitrogen is 5.19.
ZYTIGA tablets are available in 500 mg film-coated tablets and 250 mg uncoated tablets with the following inactive ingredients:
- 500 mg film-coated tablets: colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, silicified microcrystalline cellulose, and sodium lauryl sulfate. The coating, Opadry® II Purple, contains iron oxide black, iron oxide red, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
- 250 mg uncoated tablets: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate.
-
Mechanism of Action
Abiraterone acetate (ZYTIGA) is converted in vivo to abiraterone, an androgen biosynthesis inhibitor, that inhibits 17 α-hydroxylase/C17,20-lyase (CYP17). This enzyme is expressed in testicular, adrenal, and prostatic tumor tissues and is required for androgen biosynthesis.
CYP17 catalyzes two sequential reactions: 1) the conversion of pregnenolone and progesterone to their 17α-hydroxy derivatives by 17α-hydroxylase activity and 2) the subsequent formation of dehydroepiandrosterone (DHEA) and androstenedione, respectively, by C17, 20 lyase activity. DHEA and androstenedione are androgens and are precursors of testosterone. Inhibition of CYP17 by abiraterone can also result in increased mineralocorticoid production by the adrenals.
Androgen sensitive prostatic carcinoma responds to treatment that decreases androgen levels. Androgen deprivation therapies, such as treatment with GnRH agonists or orchiectomy, decrease androgen production in the testes but do not affect androgen production by the adrenals or in the tumor.
ZYTIGA decreased serum testosterone and other androgens in patients in the placebo-controlled clinical trial. It is not necessary to monitor the effect of ZYTIGA on serum testosterone levels.
Changes in serum prostate specific antigen (PSA) levels may be observed but have not been shown to correlate with clinical benefit in individual patients.
What should I tell my healthcare provider before taking Zytiga?
Before taking Zytiga, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems
- have liver problems
- have diabetes
- have a history of adrenal problems
- have a history of pituitary problems
- are receiving any other treatment for prostate cancer
- are pregnant or plan to become pregnant. Zytiga can cause harm to your unborn baby and loss of pregnancy (miscarriage). Females who are or may become pregnant should not handle Zytiga uncoated tablets or other Zytiga tablets if broken, crushed, or damaged without protection, such as gloves.
- have a partner who is pregnant or may become pregnant.
- Males who have female partners who are able to become pregnant should use effective birth control (contraception) during treatment with Zytiga and for 3 weeks after the last dose of Zytiga.
- are breastfeeding or plan to breastfeed. It is not known if Zytiga passes into your breastmilk.
Tell your healthcare provider about all the medicines you take or treatments you receive, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Zytiga can interact with many other medicines.
You should not start or stop any medicine before you talk with the healthcare provider that prescribed Zytiga.
Know the medicines you take. Keep a list of them with you to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take Zytiga?
- Take Zytiga and prednisone exactly as your healthcare provider tells you.
- Take your prescribed dose of Zytiga 1 time a day.
- Your healthcare provider may change your dose if needed.
- Do not change or stop taking your prescribed dose of Zytiga or prednisone without talking with your healthcare provider first.
- Take Zytiga on an empty stomach, at least one hour before or at least two hours after a meal. Do not take Zytiga with food. Taking Zytiga with food may cause more of the medicine to be absorbed by the body than is needed and this may cause side effects.
- Swallow Zytiga tablets whole. Do not crush or chew tablets.
- Take Zytiga tablets with water.
- If you miss a dose of Zytiga or prednisone, take your prescribed dose the following day. If you miss more than 1 dose, tell your healthcare provider right away.
- Your healthcare provider will do blood tests to check for side effects.
What are the possible side effects of Zytiga?
Zytiga may cause serious side effects including:
- High blood pressure (hypertension), low blood potassium levels (hypokalemia), fluid retention (edema), and irregular heartbeats can happen during treatment with Zytiga. This can be life threatening. To decrease the chance of this happening, you must take prednisone with Zytiga exactly as your healthcare provider tells you. Your healthcare provider will check your blood pressure, do blood tests to check your potassium levels, and check for any signs and symptoms of fluid retention every month during treatment with Zytiga.
Tell your healthcare provider if you get any of the following symptoms:- dizziness
- fast or irregular heartbeats
- feel faint or lightheaded
- headache
- confusion
- muscle weakness
- pain in your legs
- swelling in your legs or feet
- Adrenal problems may happen if you stop taking prednisone, get an infection, or are under stress.
- Severe liver problems. You may develop changes in liver function blood test. Your healthcare provider will do blood tests to check your liver before treatment with Zytiga and during treatment with Zytiga. Liver failure may occur, which can lead to death. Tell your healthcare provider right away if you notice any of the following changes:
- Increased risk of bone fracture and death when Zytiga and prednisone or prednisolone, is used in combination with a type of radiation called radium Ra 223 dichloride. Tell your healthcare provider about any other treatments you are taking for prostate cancer.
- Severe low blood sugar (hypoglycemia). Severe low blood sugar with Zytiga can happen in people who have diabetes and take certain antidiabetic medicines. You and/or your healthcare provider should check your blood sugar levels regularly during treatment with Zytiga and after you stop treatment. Your healthcare provider may also need to change the dose of your antidiabetic medicines. Signs and symptoms of low blood sugar may include.
- headache
- drowsiness
- weakness
- dizziness
- confusion
- irritability
- hunger
- fast heart beat
- sweating
- feeling jittery
The most common side effects of Zytiga include:
- feeling very tired
- joint pain
- high blood pressure
- nausea
- swelling in your legs or feet
- low blood potassium levels
- hot flushes
- diarrhea
- vomiting
- infected nose, sinuses, or throat (cold)
- cough
- headache
- low red blood cells (anemia)
- high blood cholesterol and triglycerides
- high blood sugar levels
- certain other abnormal blood tests
Zytiga may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of Zytiga. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Zytiga
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Zytiga for a condition for which it was not prescribed. Do not give Zytiga to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Zytiga that is written for health professionals.
How should I store Zytiga?
- Store Zytiga at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Zytiga and all medicines out of the reach of children.
What are the ingredients in Zytiga?
Active ingredient: abiraterone acetate
Inactive ingredients:
500 mg film-coated tablets: colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, silicified microcrystalline cellulose, and sodium lauryl sulfate. The film-coating contains iron oxide black, iron oxide red, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
250 mg uncoated tablets: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate.
For more information, call Janssen Biotech, Inc. at 1-800-526-7736 (1-800-JANSSEN) or go to www.Zytiga.com.
Label
PRINCIPAL DISPLAY PANEL – 250 MG TABLET BOTTLE LABEL
- NDC 57894-150-12
Rx only - Zytiga®
(abiraterone acetate)
tablets - 250 mg
- Each tablet contains
250 mg of abiraterone acetate. - Warning: Women who are or
may be pregnant should not handle
ZYTIGA without gloves
(see Prescribing Information). - 120 tablets
janssen

![]()
PRINCIPAL DISPLAY PANEL – 500 MG TABLET BOTTLE LABEL
- NDC 57894-195-06
- Zytiga® 500 mg
(abiraterone acetate)
Tablets - Each film-coated tablet contains
500 mg of abiraterone acetate. - Rx only
- 60 film-coated tablets
janssen 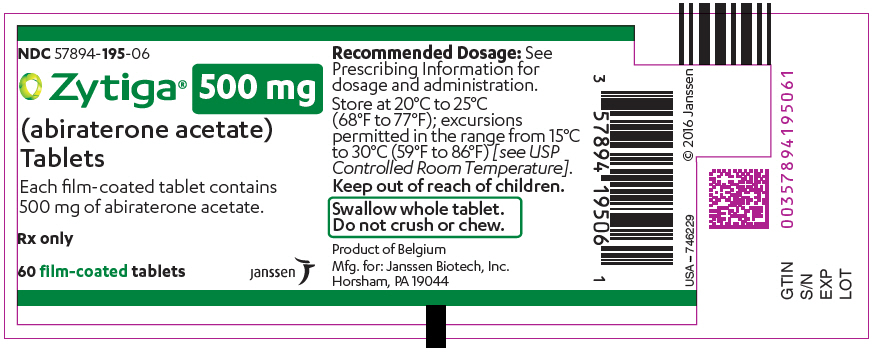
SRC: NLM .

