Xospata
Generic name: gilteritinib
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD. Last updated on june 03,2022.
What is Xospata?
Xospata is a prescription medicine used to treat adults with acute myeloid leukemia (AML) who have a FMS-like tyrosine kinase 3 (FLT3) mutation:
- when the disease has come back, or
- has not improved after previous treatment(s).
Your healthcare provider will perform a test to make sure that Xospata is right for you.
It is not known if Xospata is safe and effective in children.
Description
Gilteritinib is a kinase inhibitor. The chemical name is 2-Pyrazinecarboxamide, 6-ethyl-3-[[3-methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperidinyl] phenyl] amino]-5-[(tetrahydro-2H-pyran-4-yl) amino]-, (2E)-2-butenedioate (2:1). The molecular weight is 1221.50 and the molecular formula is (C29H44N8O3)2·C4H4O4. The structural formula is:
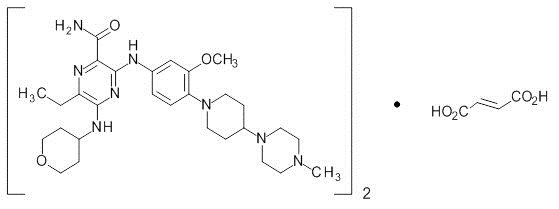
Gilteritinib fumarate is a light yellow to yellow powder or crystals that is sparingly soluble in water and very slightly soluble in anhydrous ethanol.
XOSPATA (gilteritinib) is provided as a tablet for oral administration. Each tablet contains 40 mg of gilteritinib active ingredient as free base (corresponding to 44.2 mg gilteritinib fumarate). The inactive ingredients are ferric oxide, hydroxypropyl cellulose, hypromellose, low-substituted hydroxypropyl cellulose, mannitol, magnesium stearate, polyethylene glycol, talc, and titanium dioxide.
Mechanism of Action
Gilteritinib is a small molecule that inhibits multiple receptor tyrosine kinases, including FMS-like tyrosine kinase 3 (FLT3). Gilteritinib demonstrated the ability to inhibit FLT3 receptor signaling and proliferation in cells exogenously expressing FLT3 including FLT3-ITD, tyrosine kinase domain mutations (TKD) FLT3-D835Y and FLT3-ITD-D835Y, and it induced apoptosis in leukemic cells expressing FLT3-ITD.
What is the most important information I should know about Xospata?
Xospata may cause serious side effects, including:
Differentiation Syndrome. Differentiation syndrome is a condition that affects your blood cells and may be life-threatening or lead to death if not treated. Differentiation syndrome can happen as early as 2 days after starting Xospata and during the first 3 months of treatment. Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome while taking Xospata:
- fever
- cough
- trouble breathing
- rash
- dizziness or lightheadedness
- rapid weight gain
- swelling of your arms or legs
- decreased urination
If you develop any of these symptoms of differentiation syndrome, your healthcare provider may treat you with a corticosteroid medicine and may monitor you in the hospital.
See “What are the possible side effects of Xospata?” for more information about side effects.
Who should not take Xospata?
Do not take Xospata if you are allergic to gilteritinib or any of the ingredients in Xospata. See the end of this Medication Guide for a complete list of ingredients in Xospata.
What should I tell my healthcare provider before taking Xospata?
Before taking Xospata, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including a condition called long QT syndrome.
- have problems with abnormal electrolytes such as sodium, potassium, or magnesium levels.
- are pregnant or plan to become pregnant. Xospata can cause harm to your unborn baby. You should avoid becoming pregnant during treatment with Xospata. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Xospata.
- If you are able to become pregnant, your healthcare provider may perform a pregnancy test 7 days before you start treatment with Xospata.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with Xospata and for at least 6 months after the last dose of Xospata.
- Males who have female partners that are able to become pregnant should use effective birth control (contraception) during treatment with Xospata and for at least 4 months after the last dose of Xospata.
- are breastfeeding or plan to breastfeed. It is not known if Xospata passes into your breast milk. Do not breastfeed during treatment with Xospata and for at least 2 months after the last dose of Xospata.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take Xospata?
- Take Xospata exactly as your healthcare provider tells you to. Do not change your dose or stop taking Xospata without talking to your healthcare provider.
- Take Xospata 1 time a day at about the same time each day.
- Swallow Xospata tablets whole. Do not break, crush, or chew the tablet.
- Xospata can be taken with or without food.
- If you miss a dose of Xospata, or did not take it at the usual time, take your dose as soon as possible and at least 12 hours before your next dose. Return to your normal schedule the following day. Do not take 2 doses of Xospata within 12 hours.
What are the possible side effects of Xospata?
Xospata may cause serious side effects, including:
- See “What is the most important information I should know about Xospata?”
- Posterior Reversible Encephalopathy Syndrome (PRES). If you take Xospata, you may be at risk of developing a condition involving the brain called PRES. Tell your healthcare provider right away if you have a seizure or quickly worsening symptoms such as headache, decreased alertness, confusion, reduced eyesight, blurred vision, or other visual problems. Your healthcare provider will do a test to check for PRES. Your healthcare provider will stop Xospata if you develop PRES.
- Changes in the electrical activity of your heart called QTc prolongation. QTc prolongation can cause irregular heartbeats that can be life-threatening. Your healthcare provider will check the electrical activity of your heart with a test called an electrocardiogram (ECG) before you start taking Xospata and during your treatment with Xospata. Tell your healthcare provider right away if you feel dizzy, lightheaded, or faint. The risk of QT prolongation is higher in people with low blood magnesium or low blood potassium levels. Your healthcare provider will do blood tests to check your potassium and magnesium levels before and during your treatment with Xospata.
- Inflammation of the pancreas (pancreatitis). Tell your healthcare provider right away if you have severe stomach (abdomen) pain that does not go away. This pain may happen with or without nausea and vomiting.
The most common side effects of Xospata include:
- changes in liver function tests
- joint or muscle pain
- tiredness
- fever
- pain or sores in mouth or throat
- swelling of arms or legs
- rash
- diarrhea
- shortness of breath
- nausea
- cough
- constipation
- eye problems
- headache
- dizziness
- low blood pressure
- vomiting
- decreased urination
Your healthcare provider may tell you to decrease your dose, temporarily stop, or completely stop taking Xospata if you develop certain side effects during treatment with Xospata.
These are not all of the possible side effects of Xospata.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Xospata
Medicines are sometimes prescribed for conditions not listed in a Medication Guide. Do not use Xospata for a condition for which it was not prescribed. Do not give Xospata to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Xospata that is written for healthcare professionals.
How should I store Xospata?
- Xospata comes in a child-resistant package.
- Store Xospata at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Xospata in the original container provided by your pharmacist to protect it from light, moisture and humidity.
- Keep Xospata and all medicines out of the reach of children.
What are the ingredients in Xospata?
Active ingredient: gilteritinib
Inactive ingredients: ferric oxide, hydroxypropyl cellulose, hypromellose, low-substituted hydroxypropyl cellulose, mannitol, magnesium stearate, talc, polyethylene glycol and titanium dioxide.
For more information about Xospata, call 1-800-727-7003, or visit www.XOSPATA.com.
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 40 MG TABLET BOTTLE CARTON
- NDC 0469-1425-90
- XOSPATA®
(gilteritinib) tablets
40 mg - Do not break, crush or chew tablets.
- 90 tablets
- Rx only

SRC: NLM .
