Xeloda
Generic name: capecitabine
Drug class: Antimetabolites
Medically reviewed by A Ras MD.
What is Xeloda?
Xeloda is a prescription medicine used to treat people with cancer of the colon that has spread to lymph nodes in the area close to the colon (Dukes’ C stage), after they have surgery.
It is also used to treat cancer of the colon or rectum (colorectal) that has spread to other parts of the body (metastatic), breast cancer that has spread to other parts of the body (metastatic) together with another medicine called docetaxel after treatment with certain other anti-cancer medicines have not worked, breast cancer that has spread to other parts of the body and has not improved after treatment with paclitaxel and certain other anti-cancer medicines, or who cannot receive any more treatment with certain anti-cancer medicines.
It is not known if Xeloda is safe and effective in children.
Description
XELODA (capecitabine) is a fluoropyrimidine carbamate with antineoplastic activity. It is an orally administered systemic prodrug of 5′-deoxy-5-fluorouridine (5′-DFUR) which is converted to 5-fluorouracil.
The chemical name for capecitabine is 5′-deoxy-5-fluoro-N-[(pentyloxy) carbonyl]-cytidine and has a molecular weight of 359.35. Capecitabine has the following structural formula:
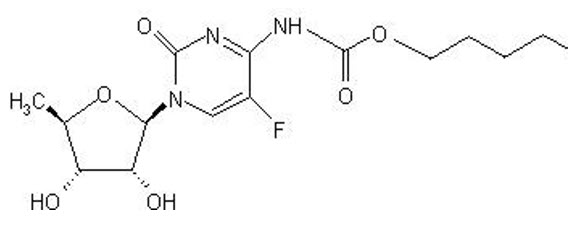
Capecitabine is a white to off-white crystalline powder with an aqueous solubility of 26 mg/mL at 20ºC.
XELODA is supplied as biconvex, oblong film-coated tablets for oral administration. Each light peach-colored tablet contains 150 mg capecitabine and each peach-colored tablet contains 500 mg capecitabine. The inactive ingredients in XELODA include: anhydrous lactose, croscarmellose sodium, hydroxypropyl methylcellulose, microcrystalline cellulose, magnesium stearate and purified water. The peach or light peach film coating contains hydroxypropyl methylcellulose, talc, titanium dioxide, and synthetic yellow and red iron oxides.
Mechanism of Action
Enzymes convert capecitabine to 5-fluorouracil (5-FU) in vivo. Both normal and tumor cells metabolize 5-FU to 5-fluoro-2′-deoxyuridine monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). These metabolites cause cell injury by two different mechanisms. First, FdUMP and the folate cofactor, N5-10-methylenetetrahydrofolate, bind to thymidylate synthase (TS) to form a covalently bound ternary complex. This binding inhibits the formation of thymidylate from 2′-deoxyuridylate. Thymidylate is the necessary precursor of thymidine triphosphate, which is essential for the synthesis of DNA, so that a deficiency of this compound can inhibit cell division. Second, nuclear transcriptional enzymes can mistakenly incorporate FUTP in place of uridine triphosphate (UTP) during the synthesis of RNA. This metabolic error can interfere with RNA processing and protein synthesis.
What is the most important information I should know about Xeloda?
Xeloda can cause serious side effects, including:
- Xeloda can interact with blood thinner medicines, such as warfarin (Coumadin). Taking Xeloda with these medicines can cause changes in how fast your blood clots and can cause bleeding that can lead to death. This can happen as soon as a few days after you start taking Xeloda, or later during treatment, and possibly even within 1 month after you stop taking Xeloda. Your risk may be higher because you have cancer, and if you are over 60 years of age.
- Before taking Xeloda, tell your healthcare provider if you are taking warfarin (Coumadin) or another blood thinner medicine.
- If you take warfarin (Coumadin) or another blood thinner that is like warfarin (Coumadin) during treatment with Xeloda, your healthcare provider should do blood tests often, to check how fast your blood clots during and after you stop treatment with Xeloda. Your healthcare provider may change your dose of the blood thinner medicine if needed.
See “What are the possible side effects of Xeloda?” for more information about side effects.
Who should not take Xeloda?
Do not take Xeloda if you:
- have severe kidney problems.
- are allergic to capecitabine, 5-fluorouracil, or any of the ingredients in Xeloda. See the end of this leaflet for a complete list of ingredients in Xeloda.
Talk to your healthcare provider before taking Xeloda if you are not sure if you have any of the conditions listed above.
What should I tell my healthcare provider before taking Xeloda?
Before taking Xeloda, tell your healthcare provider about all your medical conditions, including if you:
See “What is the most important information I should know about Xeloda?”
- have had heart problems.
- have kidney or liver problems.
- have been told that you lack the enzyme DPD (dihydropyrimidine dehydrogenase).
- are pregnant or plan to become pregnant. Xeloda can harm your unborn baby. Your healthcare provider should do a pregnancy test before you start treatment with Xeloda. Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with Xeloda.
- Females who are able to become pregnant should use effective birth control during treatment and for 6 months after the final dose. Talk to your healthcare provider about birth control choices that may be right for you during treatment with Xeloda.
- Males who have female partners who are able to become pregnant should use effective birth control during treatment and for 3 months after the final dose.
- are breastfeeding or plan to breastfeed. It is not known if Xeloda passes into your breast milk. Do not breastfeed during treatment with Xeloda and for 2 weeks after the final dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Xeloda may affect the way other medicines work, and other medicines may affect the way Xeloda works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Xeloda?
- Take Xeloda exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much Xeloda to take and when to take it.
- Take Xeloda 2 times a day, 1 time in the morning and 1 time in the evening.
- Take Xeloda within 30 minutes after finishing a meal.
- Swallow Xeloda tablets whole with water. Do not crush or cut Xeloda tablets. If you cannot swallow Xeloda tablets whole, tell your healthcare provider.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Xeloda if you develop side effects.
- If you take too much Xeloda, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Xeloda?
Xeloda may cause serious side effects including:
See “What is the most important information I should know about Xeloda?”
- Diarrhea. Diarrhea is common with Xeloda and can sometimes be severe. Stop taking Xeloda and call your healthcare provider right away if the number of bowel movements you have in a day increases by 4 or more than is usual for you. Ask your healthcare provider about what medicines you can take to treat your diarrhea. If you have severe bloody diarrhea with severe abdominal pain and fever, call your healthcare provider or go to the nearest hospital emergency room right away.
- Heart problems. Xeloda can cause heart problems including: heart attack and decreased blood flow to the heart, chest pain, irregular heartbeats, changes in the electrical activity of your heart seen on an electrocardiogram (ECG), problems with your heart muscle, heart failure, and sudden death. Stop taking Xeloda and call your healthcare provider right away if you get any of the following symptoms:
- chest pain
- feeling faint
- sudden weight gain
- shortness of breath
- irregular heartbeats or skipping beats
- swollen ankles or legs
- Loss of too much body fluid (dehydration) and kidney failure. Dehydration can happen with Xeloda and may cause sudden kidney failure that can lead to death. You are at higher risk if you have kidney problems before taking Xeloda and also take other medicines that can cause kidney problems.
- Nausea, and vomiting are common with Xeloda. If you lose your appetite, feel weak, and have nausea, vomiting, or diarrhea, you can quickly become dehydrated.
- Stop taking Xeloda and call your doctor right away if you:
- vomit 2 or more times in a day.
- are only able to eat or drink a little now and then, or not at all due to nausea.
- have diarrhea. See above.
- Serious skin and mouth reactions.
- Xeloda can cause serious skin reactions that may lead to death. Tell your Healthcare provider right away if you develop a skin rash, blisters and peeling of your skin. Your healthcare provider may tell you to stop taking Xeloda if you have a serious skin reaction. Do not take Xeloda again if this happens.
- Xeloda can also cause “hand and foot syndrome.” Hand and foot syndrome is common with Xeloda and can cause you to have numbness and changes in sensation in your hands and feet, or cause redness, pain, swelling of your hands and feet. Stop taking Xeloda and call your healthcare provider right away if you have any of these symptoms and you are not able to do your usual activities.
- Hand and foot syndrome can lead to loss of fingerprints which could impact your identification.
- you may get sores in your mouth or on your tongue when taking Xeloda. Stop taking Xeloda and call your healthcare provider if you get painful redness, swelling, or ulcers in your mouth and tongue, or if you are having problems eating.
- Increased level of bilirubin in your blood and liver problems. Increased bilirubin in your blood is common with Xeloda. Your healthcare provider will check you for these problems during treatment with Xeloda.
- Decreased white blood cells, platelets, and red blood cell counts. Your healthcare provider will do blood tests during treatment with Xeloda to check your blood cell counts. If your white blood cell count is very low, you are at increased risk for infection. Call your healthcare provider right away if you develop a fever of 100.5°F or greater or have other signs and symptoms of infection.
People 80 years of age or older may be more likely to develop severe or serious side effects with Xeloda.
The most common side effects of Xeloda include:
- diarrhea
- hand and foot syndrome
- nausea
- vomiting
- stomach-area (abdominal) pain
- weakness and tiredness
- increased amounts of red blood cell breakdown products (bilirubin) in your blood
Xeloda may cause fertility problems in females and males. This may affect the ability to have a child. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of Xeloda.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Xeloda
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Xeloda for a condition for which it was not prescribed. Do not give Xeloda to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Xeloda that is written for health professionals.
How should I store Xeloda?
- Store Xeloda at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Xeloda in a tightly closed container.
- Ask your healthcare provider or pharmacist how to safely throw away any unused Xeloda.
Keep Xeloda and all medicines out of the reach of children.
What are the ingredients in Xeloda?
Active ingredient: capecitabine
Inactive ingredients: anhydrous lactose, croscarmellose sodium, hydroxypropyl methylcellulose, microcrystalline cellulose, magnesium stearate and purified water. The peach or light peach film coating contains hydroxypropyl methylcellulose, talc, titanium dioxide, and synthetic yellow and red iron oxides.
Label
PRINCIPAL DISPLAY PANEL – 150 MG TABLET BOTTLE CARTON
- NDC 0004-1100-20
- Xeloda®
(capecitabine)
Tablets - 150 mg
- Each tablet contains 150 mg
capecitabine. - Rx only
- 60 tablets
Genentech - 10210268

PRINCIPAL DISPLAY PANEL – 500 MG TABLET BOTTLE CARTON
- NDC 0004-1101-50
- Xeloda®
(capecitabine)
Tablets - 500 mg
- Each tablet contains 500 mg
capecitabine. - Rx only
- 120 tablets
Genentech - 10210269

SRC: NLM .
