Xalkori
Generic name: crizotinib
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD.
What is Xalkori?
Xalkori is a prescription medicine that is used to treat people with non-small cell lung cancer (NSCLC) that has spread to other parts of the body and is caused by a defect in either a gene called ALK (anaplastic lymphoma kinase) or a gene called ROS1.
It is also used in children 1 year of age and older and young adults when your ALCL with a defect in a gene called ALK has returned, or you have tried a treatment and it did not work or is no longer working.
It is not known if Xalkori is safe and effective in children under 12 months of age or in older adults with ALCL.
Description
Crizotinib is a kinase inhibitor. The molecular formula for crizotinib is C21H22Cl2FN5O and the molecular weight is 450.34 daltons. Crizotinib is described chemically as (R)-3-[1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-amine.
The chemical structure of crizotinib is shown below:
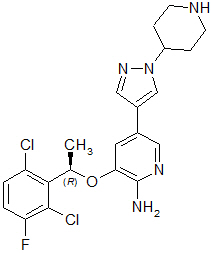
Crizotinib is a white to pale-yellow powder with a pKa of 9.4 (piperidinium cation) and 5.6 (pyridinium cation). The solubility of crizotinib in aqueous media decreases over the range pH 1.6 to pH 8.2 from greater than 10 mg/mL to less than 0.1 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7.4 is 1.65.
XALKORI (crizotinib) for oral administration is supplied as printed hard-shell capsules containing 250 mg or 200 mg of crizotinib together with colloidal silicon dioxide, microcrystalline cellulose, anhydrous dibasic calcium phosphate, sodium starch glycolate, magnesium stearate, and hard gelatin capsule shells as inactive ingredients.
The pink opaque capsule shell components contain gelatin, titanium dioxide, and red iron oxide. The white opaque capsule shell components contain gelatin and titanium dioxide. The printing ink contains shellac, propylene glycol, strong ammonia solution, potassium hydroxide, and black iron oxide.
Mechanism of Action
Crizotinib is an inhibitor of receptor tyrosine kinases including ALK, Hepatocyte Growth Factor Receptor (HGFR, c-Met), ROS1 (c-ros), and Recepteur d’Origine Nantais (RON). Translocations can affect the ALK gene resulting in the expression of oncogenic fusion proteins. The formation of ALK fusion proteins results in activation and dysregulation of the gene’s expression and signaling which can contribute to increased cell proliferation and survival in tumors expressing these proteins. Crizotinib demonstrated concentration-dependent inhibition of ALK, ROS1, and c-Met phosphorylation in cell-based assays using tumor cell lines and demonstrated antitumor activity in mice bearing tumor xenografts that expressed echinoderm microtubule-associated protein-like 4 (EML4)- or nucleophosmin (NPM)-ALK fusion proteins or c-Met.
In vitro, crizotinib induced apoptosis and inhibited proliferation and ALK-mediated signaling in ALCL-derived cell lines (containing NPM-ALK) at clinically achievable exposures. In vivo data obtained in an ALCL-derived mouse model showed complete regression of the tumor at a dose of 100 mg/kg once daily.
What is the most important information I should know about Xalkori?
Xalkori may cause serious side effects, including:
- Liver problems. Xalkori may cause life-threatening liver injury that may lead to death. Your healthcare provider should do blood tests to check your liver every 2 weeks during the first 2 months of treatment with Xalkori, then once a month and as recommended by your healthcare provider during treatment. Tell your healthcare provider right away if you develop any of the following new or worsening symptoms:
- Lung problems (pneumonitis). Xalkori may cause life-threatening lung problems that may lead to death. Symptoms may be similar to those symptoms from lung cancer. Tell your healthcare provider right away if you have any new or worsening symptoms, including:
- trouble breathing or shortness of breath
- cough with or without mucous
- fever
- Heart problems. Xalkori may cause very slow, very fast, or abnormal heartbeats. Your healthcare provider may check your pulse rate and blood pressure regularly during treatment with Xalkori. Tell your healthcare provider right away if you feel dizzy or faint or have abnormal heartbeats. Tell your healthcare provider if you take any heart or blood pressure medicines.
- Severe vision problems. Vision problems are common with Xalkori. These problems usually happen within 1 week of starting treatment with Xalkori. Vision problems with Xalkori can be severe and may cause partial or complete loss of vision in one or both eyes. Your healthcare provider may hold or permanently stop your treatment with Xalkori and refer you to an eye specialist if any vision problems develop during treatment with Xalkori. Tell your healthcare provider right away if you have any new vision problems, loss of vision or any change in vision, including:
- double vision
- seeing flashes of light
- blurry vision
- light hurting your eyes
- new or increased floaters
In addition, for people taking Xalkori to treat anaplastic large cell lymphoma (ALCL): Your healthcare provider may refer you to an eye specialist before starting Xalkori, and within 1 month of starting Xalkori to check for vision problems. You should have an eye examination every 3 months during treatment with Xalkori and more often if there are any new vision problems.
- Severe stomach, intestine, and mouth (gastrointestinal) problems in people with ALCL. Xalkori may cause severe diarrhea, nausea, vomiting, or mouth sores. Tell your healthcare provider right away if problems with swallowing, vomiting, or diarrhea develop during treatment with Xalkori.
- Your healthcare provider may give medicines as needed to prevent or treat diarrhea, nausea, and vomiting.
- Your healthcare provider may recommend drinking more fluids or may prescribe electrolyte supplements or other kinds of nutritional support if severe symptoms develop.
See “What are the possible side effects of Xalkori?” for more information about side effects.
What should I tell my healthcare provider before taking Xalkori?
Before taking Xalkori, tell your healthcare provider about all of your medical conditions, including if you:
- have liver or kidney problems
- have lung problems
- have heart problems, including a condition called long QT syndrome
- have vision or eye problems
- are pregnant, or plan to become pregnant. Xalkori can harm the unborn baby.
Females who are able to become pregnant:- Your healthcare provider will check to see if you are pregnant before starting treatment with Xalkori.
- Effective birth control (contraception) should be used during treatment with Xalkori and for at least 45 days after the final dose of Xalkori.
- Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with Xalkori.
Males who have female partners who can become pregnant:
- You should use condoms during treatment with Xalkori and for at least 90 days after the final dose of Xalkori.
- are breastfeeding or plan to breastfeed. It is not known if Xalkori passes into the breast milk. Do not breastfeed during treatment with Xalkori and for 45 days after the final dose. Talk to your healthcare provider about the best way to feed the baby during this time.
Tell your healthcare provider about the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements.
How should I take Xalkori?
- Xalkori should be taken exactly as prescribed by your healthcare provider.
- Xalkori capsules should be swallowed whole.
- Xalkori may be taken with or without food.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Xalkori if you have certain side effects. Do not change the dose or stop treatment with Xalkori unless your healthcare provider tells you to.
- If a dose of Xalkori is missed, it should be taken as soon as you remember. If it is close to the time of the next dose (within 6 hours), the missed dose should be skipped, and the next dose should be taken at the regular time.
- If you vomit after taking a dose of Xalkori, do not take an extra dose. The next dose should be taken at the regular time.
- Xalkori should be given to children under adult supervision.
What should I avoid while taking Xalkori?
- Do not drink grapefruit juice, eat grapefruit or take supplements containing grapefruit extract during treatment with Xalkori. These may increase the amount of Xalkori in the blood.
- Xalkori can cause changes in vision, dizziness, and tiredness. Do not drive or operate machinery if you have any of these symptoms.
What are the possible side effects of Xalkori?
Xalkori may cause serious side effects, including:
- See “What is the most important information I should know about Xalkori?”
The most common side effects of Xalkori in people with NSCLC include:
- vision problems
- nausea, diarrhea, or vomiting
- swelling of your hands, feet, face, and eyes
- constipation
- increased liver function blood tests
- tiredness
- decreased appetite
- upper respiratory infection
- dizziness
- feeling of numbness or tingling in your arms or legs
The most common side effects of Xalkori in people with ALCL include:
- diarrhea, vomiting, or nausea
- vision problems
- headache
- muscle and joint pain
- mouth sores
- tiredness
- decreased appetite
- fever
- stomach-area (abdominal) pain
- cough
- itchy skin
- low blood counts
- abnormal liver tests
- low levels of electrolytes
- abnormal kidney tests
- low and high blood sugar levels
Xalkori may cause fertility problems in females and males, which may affect the ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of Xalkori. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Xalkori
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Xalkori for a condition for which it was not prescribed. Do not give Xalkori to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about Xalkori that is written for health professionals.
How should I store Xalkori?
- Store Xalkori at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Xalkori and all medicines out of the reach of children.
What are the ingredients in Xalkori?
Active ingredient: crizotinib
Inactive ingredients: colloidal silicon dioxide, microcrystalline cellulose, anhydrous dibasic calcium phosphate, sodium starch glycolate, and magnesium stearate.
Pink opaque capsule shell contains: gelatin, titanium dioxide, and red iron oxide.
White opaque capsule shell contains: gelatin and titanium dioxide.
Printing ink contains: shellac, propylene glycol, strong ammonia solution, potassium hydroxide, and black iron oxide.
Label
PRINCIPAL DISPLAY PANEL – 250 MG CAPSULE BOTTLE LABEL
- ALWAYS DISPENSE ENCLOSED MEDICATION
GUIDE TO EACH PATIENT - Pfizer
NDC 0069-8140-20 - XALKORI®
(crizotinib) capsules
250 mg - Swallow capsule whole
- 60 Capsules
Rx only


SRC: NLM .
