Vuity
Generic name: pilocarpine hydrochloride
Dosage form: ophthalmic solution
Medically reviewed by A Ras MD. Last updated on April 9, 2022
What is Vuity?
Vuity is being used to treat glaucoma or ocular hypertension (high pressure inside the eye).
Vuity is a treatment for a degenerative age-related disorder that impairs the eyes’ ability to focus on close objects (presbyopia).
Vuity can be used for a variety of other things that aren’t included in this drug guide.
1 INDICATIONS AND USAGE
VUITY is indicated for the treatment of presbyopia in adults.
2 DOSAGE AND ADMINISTRATION
The recommended dosage of VUITY is one drop in each eye once daily.
If more than one topical ophthalmic product is being used, the products should be administered at least 5 minutes apart.
3 DOSAGE FORMS AND STRENGTHS
VUITY (pilocarpine hydrochloride ophthalmic solution) is provided as a 1.25% solution (12.5 mg/mL).
4 CONTRAINDICATIONS
VUITY is contraindicated in patients with known hypersensitivity to the active ingredient or to any of the excipients.
5 WARNINGS AND PRECAUTIONS
5.1 Poor Illumination
Patients should be advised to exercise caution in night driving and other hazardous occupations in poor illumination. In addition, miotics may cause accommodative spasm. Patients should be advised not to drive or use machinery if vision is not clear.
5.2 Risk of Retinal Detachment
Rare cases of retinal detachment have been reported with other miotics when used in susceptible individuals and those with pre-existing retinal disease. Patients should be advised to seek immediate medical care with sudden onset of vision loss.
5.3 Iritis
VUITY is not recommended to be used when iritis is present because adhesions (synechiae) may form between the iris and the lens.
5.4 Use with Contact Lenses
Contact lens wearers should be advised to remove their lenses prior to the instillation of VUITY and to wait 10 minutes after dosing before reinserting their contact lenses.
5.5 Potential for Eye Injury or Contamination
To prevent eye injury or contamination, care should be taken to avoid touching the dispensing bottle to the eye or to any other surface.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Hypersensitivity [see Contraindications (4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
VUITY was evaluated in 375 patients with presbyopia in two randomized, double-masked, vehicle-controlled studies (GEMINI 1 and GEMINI 2) of 30 days duration. The most common adverse reactions reported in >5% of patients were headache and conjunctival hyperemia. Ocular adverse reactions reported in 1-5% of patients were blurred vision, eye pain, visual impairment, eye irritation, and increased lacrimation.¶
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of VUITY administration in pregnant women to inform a drug-associated risk. Oral administration of pilocarpine to pregnant rats throughout organogenesis and lactation did not produce adverse effects at clinically relevant doses.
Data
Human Data
No adequate and well-controlled trials of VUITY have been conducted in pregnant women. In a retrospective case series of 15 women with glaucoma, 4 patients used ophthalmic pilocarpine either pre-pregnancy, during pregnancy or postpartum. There were no adverse effects observed in patients or in their infants.
Animal Data
In embryofetal development studies, oral administration of pilocarpine to pregnant rats throughout organogenesis produced maternal toxicity, skeletal anomalies and reduction in fetal body weight at 90 mg/kg/day (approximately 970-fold higher than the maximum recommended human ophthalmic dose [MRHOD] of 0.015 mg/kg/day, on a mg/m2 basis).
In a peri-/postnatal study in rats, oral administration of pilocarpine during late gestation through lactation increased stillbirths at a dose of 36 mg/kg/day (approximately 390-fold higher than the MRHOD). Decreased neonatal survival and reduced mean body weight of pups were observed at ≥18 mg/kg/day (approximately 200 times the recommended human daily dose of VUITY).
8.2 Lactation
Risk Summary
There is no information regarding the presence of pilocarpine in human milk, the effects on the breastfed infants, or the effects on milk production to inform risk of VUITY to an infant during lactation.
Pilocarpine and/or its metabolites are excreted in the milk of lactating rats. Systemic levels of pilocarpine following topical ocular administration are low [see Clinical Pharmacology (12.3)], and it is not known whether measurable levels of pilocarpine would be present in maternal milk following topical ocular administration.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VUITY and any potential adverse effects on the breastfed child from VUITY.
Data
Animal Data
Following a single oral administration of 14C-pilocarpine to lactating rats, the radioactivity concentrations in milk were similar to those in plasma.
8.4 Pediatric Use
Presbyopia does not occur in the pediatric population.
8.5 Geriatric Use
Clinical studies of VUITY did not include subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience with ophthalmic pilocarpine solutions have not identified overall differences in safety between elderly and younger patients.
10 OVERDOSAGE
Systemic toxicity following topical ocular administration of pilocarpine is rare, but occasionally patients who are sensitive may develop sweating and gastrointestinal overactivity. Accidental ingestion can produce sweating, salivation, nausea, tremors and slowing of the pulse and a decrease in blood pressure. In moderate overdosage, spontaneous recovery is to be expected and is aided by intravenous fluids to compensate for dehydration. For patients demonstrating severe poisoning, atropine, the pharmacologic antagonist to pilocarpine, should be used.
11 DESCRIPTION
VUITY (pilocarpine hydrochloride ophthalmic solution) 1.25% is a cholinergic muscarinic receptor agonist prepared as an isotonic, colorless, sterile ophthalmic solution containing 1.25% of pilocarpine hydrochloride. The chemical name for pilocarpine hydrochloride is (3S,4R)-3-ethyl-4-[(1-methyl-1H-imidazol-5-yl)methyl]oxolan-2-one hydrochloride. Its molecular weight is 244.72 and its molecular formula is C11H16N2O2 · HCl. Its structural formula is:

Each mL of VUITY contains pilocarpine hydrochloride 1.25% (12.5 mg) as the active ingredient, equivalent to 1.06% (10.6 mg) pilocarpine free-base. Preservative is: benzalkonium chloride 0.0075%. Inactive ingredients in the ophthalmic solution are: boric acid, sodium citrate dihydrate, sodium chloride, purified water, and may also include hydrochloric acid and/or sodium hydroxide for pH adjustment to between 3.5 and 5.5, if necessary.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pilocarpine hydrochloride is a cholinergic muscarinic agonist which activates muscarinic receptors located at smooth muscles such as the iris sphincter muscle and ciliary muscle. VUITY contracts the iris sphincter muscle, constricting the pupil to improve near and intermediate visual acuity while maintaining some pupillary response to light. VUITY also contracts the ciliary muscle and may shift the eye to a more myopic state.
12.3 Pharmacokinetics
Systemic exposure to pilocarpine was evaluated in 22 participants with presbyopia who were administered 1 drop of VUITY in each eye once daily for 30 days. The mean Cmax and AUC0-t,ss values on Day 30 were 1.95 ng/mL and 4.14 ng·hr/mL, respectively. The median Tmax value on Day 30 was 0.3 hours postdose with a range from 0.2 to 0.5 hours postdose.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Pilocarpine did not induce tumors in mice at any dosage level studied (up to 30 mg/kg/day; approximately 160-times the MRHOD). In rats, an oral dose of 18 mg/kg/day (approximately 200 times the MRHOD), resulted in a statistically significant increase in the incidence of benign pheochromocytomas in both male and female rats, and a statistically significant increase in the incidence of hepatocellular adenomas in female rats.
Mutagenesis
Pilocarpine did not show any potential to cause genetic toxicity in a series of studies that included: 1) bacterial assays (Salmonella and E. coli) for reverse gene mutations; 2) an in vitro chromosome aberration assay in a Chinese hamster ovary cell line; 3) an in vivo chromosome aberration assay (micronucleus test) in mice; and 4) a primary DNA damage assay (unscheduled DNA synthesis) in rat hepatocyte primary cultures.
Impairment of Fertility
Pilocarpine oral administration to male and female rats at a dosage of 18 mg/kg/day (200 times the recommended human daily dose) resulted in impaired reproductive function, including reduced fertility, decreased sperm motility, and morphologic evidence of abnormal sperm. It is unclear whether the reduction in fertility was due to effects on males, females, or both. In dogs, exposure to pilocarpine at a dosage of 3 mg/kg/day for 6 months resulted in evidence of impaired spermatogenesis (approximately 110 times the recommended human daily dose).
14 CLINICAL STUDIES
The efficacy of VUITY for the treatment of presbyopia was demonstrated in two 30‐Day Phase 3, randomized, double‐masked, vehicle‐controlled studies, namely GEMINI 1 (NCT03804268) and GEMINI 2 (NCT03857542). A total of 750 participants aged 40 to 55 years old with presbyopia were randomized (375 to VUITY group) in two studies and participants were instructed to administer one drop of VUITY or vehicle once daily in each eye.
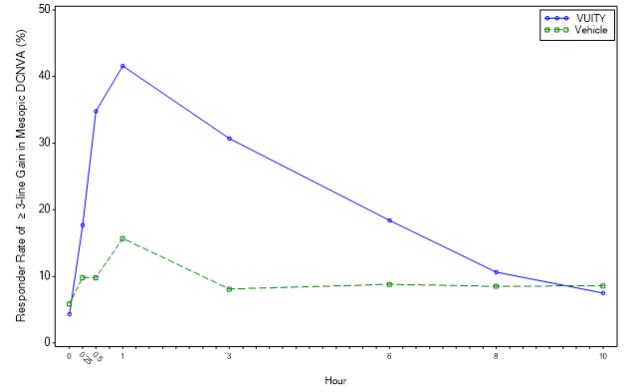
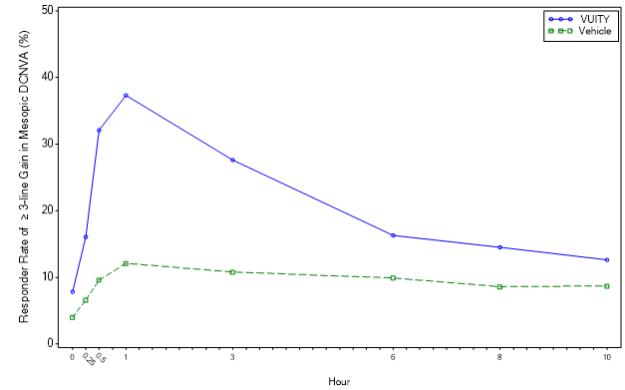
In both studies, the proportion of participants gaining 3 lines or more in mesopic, high contrast, binocular distance corrected near visual acuity (DCNVA), without losing more than 1 line (5 letters) of corrected distance visual acuity (CDVA) with the same refractive correction was statistically significantly greater in the VUITY group compared to the vehicle group at Day 30, Hour 3 (see Table 1).
| GEMINI 1 | GEMINI 2 | |||||
| VUITY N=163 |
Vehicle N=160 |
p-value | VUITY N=212 |
Vehicle N=215 |
p-value | |
| Proportion of participants gaining 3-lines or more in mesopic DCNVA, without losing more than 1 line (5 letters) of CDVA at Day 30, Hour 3 | 31% | 8% | p<0.01 | 26% | 11% | p<0.01 |
Figures 1 and 2 present the proportion of participants who gained 3-lines or more in mesopic DCNVA at Day 30.
| Figure 1: Proportion of Participants Achieving 3-Lines or More Improvement in Mesopic, High Contrast, Binocular DCNVA at Day 30 in GEMINI 1 (Intent-to-Treat Population)
|
| Figure 2: Proportion of Participants Achieving 3-lines or More Improvement in Mesopic, High Contrast, Binocular DCNVA at Day 30 in GEMINI 2 (Intent-to-Treat population)
|
How Supplied/Storage and Handling
VUITY is supplied as a sterile ophthalmic solution in colorless low density polyethylene (LDPE) ophthalmic dispenser bottles and tips, with dark green high impact polystyrene caps as follows:
| 2.5 mL fill in 5 mL bottle (Box containing 1 bottle) | NDC 0074-7098-01 |
| 2.5 mL fill in 5 mL bottle (Box containing 3 bottles) | NDC 0074-7098-03 |
| 2.5 mL fill in 5 mL bottle (Carton) | NDC 0074-7098-04 |
Storage
Store at 15°C to 25°C (59°F to 77°F). After opening, VUITY can be used until the expiration date on the bottle.
17 PATIENT COUNSELING INFORMATION
Night Driving
Caution is advised with night driving and when hazardous activities are undertaken in poor illumination. [see Warnings and Precautions (5.1)]
Accommodative Spasm
Temporary problems when changing focus between near objects and distant objects may occur. Advise patients not to drive or use machinery if vision is not clear. [see Warnings and Precautions (5.1)]
When to Seek Physician Advice
Advise patients to seek immediate medical care with sudden onset of vision loss. [see Warnings and Precautions (5.2)]
Contact Lens Wear
Contact lens should be removed prior to the instillation of VUITY. Wait 10 minutes after dosing before reinserting contact lenses. [see Warnings and Precautions (5.4)]
Avoiding Contamination of the Product
Do not touch dropper tip to any surface, as this may contaminate the contents. [see Warnings and Precautions (5.5)]
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic medication is being used, the medicines must be administered at least 5 minutes apart.
PRINCIPAL DISPLAY PANEL
- NDC 0074-7098-01
- Vuity™ (pilocarpine HCI ophthalmic solution) 1.25%
- Contains one 2.5 mL bottle
- Rx Only
- Sterile
- For topical application in the eye
- 1 x 2.5 mL
- Allergan™
- An AbbVie company

PRINCIPAL DISPLAY PANEL
- NDC 0074-7098-03
- Vuity™
- (pilocarpine HCI ophthalmic solution) 1.25%
- Contains one 2.5 mL bottle
- Rx Only
- Sterile
- For topical application in the eye
- 3 x 2.5 mL
- Allergan™
- An AbbVie company

PRINCIPAL DISPLAY PANEL
- NDC 0074-7098-04
- Vuity™ (pilocarpine HCI ophthalmic solution) 1.25%
- Rx Only
- Sterile
- 2.5 mL
- For topical Application in the Eye
- Allergan™
- An AbbVie company

PRINCIPAL DISPLAY PANEL
- NDC 0074-7098-05
- Professional Sample Not for Resale
- Vuity™ (pilocarpine HCI ophthalmic solution) 1.25%
- Rx Only
- Sterile
- 1.5 mL
- For topical Application in the Eye
- Allergan™
- An AbbVie company