Tegsedi
Generic name: inotersen
Drug class: Miscellaneous metabolic agents
Medically reviewed by A Ras MD.
What is Tegsedi?
Tegsedi is a medicine used to treat the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. It is not known if Tegsedi is safe and effective in children.
Description
Inotersen is an antisense oligonucleotide (ASO) inhibitor of human transthyretin (TTR) protein synthesis.
Tegsedi contains inotersen sodium as the active ingredient. Inotersen sodium is a white to pale yellow solid and it is freely soluble in water and in phosphate buffer (pH 7.5 to 8.5). The chemical name of inotersen sodium is DNA, d(P-thio)([2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rC-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]rG-G-T-T-A-m5C-A-T-G-A-A-[2′-O-(2-methoxyethyl)]rA-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rC-[2′-O-(2-methoxyethyl)]m5rC-[2′-O-(2-methoxyethyl)]m5rC). The molecular formula of inotersen sodium is C230H299N69 Na19O121P19S19 and the molecular weight is 7600.73 Da. It has the following structural formula:
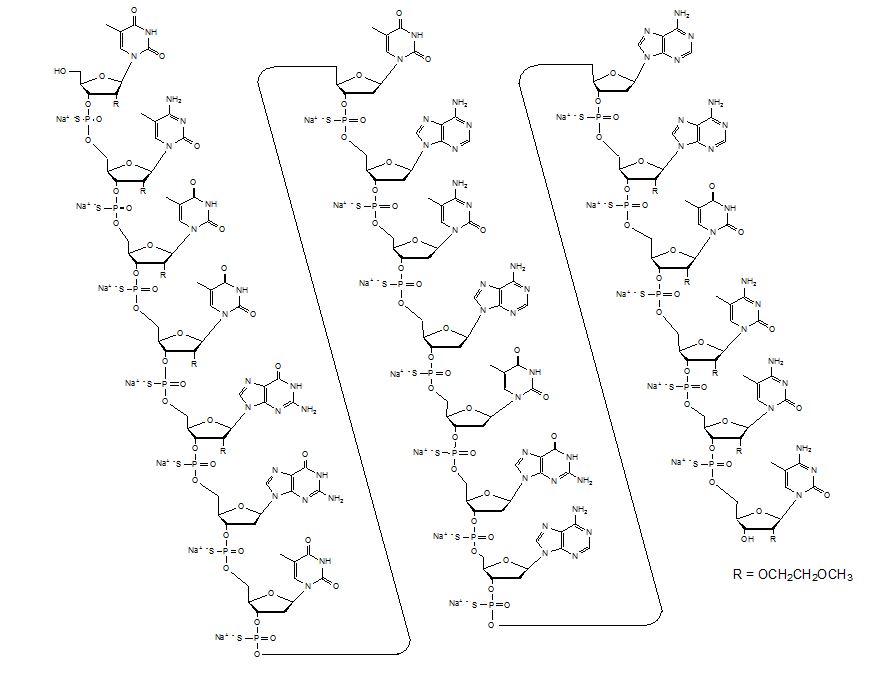
The molecular formula of inotersen free base is C230H318N69O121P19S19 and its molecular weight is 7183.08.
Tegsedi is a sterile, preservative-free, aqueous solution for subcutaneous injection. It is supplied in a prefilled syringe (PFS). Each PFS contains 1.5 mL of solution containing 284 mg inotersen (equivalent to 300 mg inotersen sodium salt) TEGSEDI is formulated in Water for Injection and may include hydrochloric acid and/or sodium hydroxide for pH adjustment to 7.5-8.5.
Mechanism of Action
Inotersen is an antisense oligonucleotide that causes degradation of mutant and wild-type TTR mRNA through binding to the TTR mRNA, which results in a reduction of serum TTR protein and TTR protein deposits in tissues.
What is the most important information I should know about Tegsedi?
Tegsedi can cause serious side effects, including:
- low platelet counts (thrombocytopenia). Tegsedi may cause the number of platelets in your blood to be reduced. This is a common side effect of Tegsedi. When your platelet count is too low, your body cannot form clots. You could have serious bleeding that could lead to death. Call your healthcare provider immediately if you have:
- unusual bruising or a rash of tiny reddish-purple spots, often on the lower legs
- bleeding from skin cuts that does not stop or oozes
- bleeding from your gums or nose
- blood in your urine or stools
- bleeding into the whites of your eyes
- sudden severe headaches or neck stiffness
- vomiting or coughing up blood
- abnormal or heavy periods (menstrual bleeding)
- kidney inflammation (glomerulonephritis).Your kidneys may stop working properly. Glomerulonephritis can lead to severe kidney damage and kidney failure that needs dialysis. Call your healthcare provider immediately if you have:
- puffiness or swelling in your face, feet or hands
- new onset or worsening shortness of breath and coughing
- blood in your urine or brown urine
- foamy urine (proteinuria)
- passed less urine than usual
Your healthcare provider will do laboratory tests to check your platelet count and kidneys before you start Tegsedi and while you are using it. Your healthcare provider should also do laboratory tests for 8 weeks after you stop Tegsedi. It is important that you make sure you get these laboratory tests done.
- Because of the risk of serious bleeding caused by low platelet counts and because of the risk of kidney problems, Tegsedi is available only through a restricted program called the Tegsedi Risk Evaluation and Mitigation (REMS) Program.
- Before you begin using Tegsedi, you must enroll in the Tegsedi REMS Program. Talk to your healthcare provider about how to enroll in the Tegsedi REMS Program.
- You must agree to get your laboratory testing done while you are in the Tegsedi REMS Program.
- You can only get Tegsedi from a certified pharmacy that participates in the Tegsedi REMS Program. Your healthcare provider can give you information on how to find a certified pharmacy.
- For more information, including a list of certified pharmacies go to www.TEGSEDIREMS.com or call 1-844-483-4736.
Who should not use Tegsedi?
Do not use Tegsedi if you have:
- a platelet count that is low.
- had kidney inflammation (glomerulonephritis) caused by Tegsedi.
- had an allergic reaction to inotersen or any of the ingredients in Tegsedi. See the end of this Medication Guide for a complete list of ingredients in Tegsedi.
What should I tell my healthcare provider before using Tegsedi?
Before you start using Tegsedi, tell your healthcare provider about all your medical conditions, including if you:
- have or had bleeding problems
- have or had kidney problems
- have received a liver transplant
- are pregnant or plan to become pregnant. It is not known if Tegsedi can harm your unborn baby.
- There is a registry for women who become pregnant during treatment with Tegsedi. If you become pregnant while taking Tegsedi, talk to your healthcare provider about registering with the Tegsedi Pregnancy Exposure Registry. The purpose of this registry is to collect information about your health and your baby’s health. You can get more information about this registry by calling: 1-877-465-7510, emailing: TegsediPREGNANCY@UBC.COM, or visiting online at: WWW.TegsediPREGNANCYSTUDY.COM.
- are breastfeeding or plan to breastfeed. It is not known if Tegsedi can pass into your breast milk or harm your baby. Talk with your healthcare provider about the best way to feed your baby while you are using Tegsedi.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
- Vitamin A or beta-carotene supplements. Your healthcare provider should tell you to take vitamin A, but only take the amount they tell you to take.
- blood thinners (anticoagulants) or medicines that affect blood clotting.
- Ask your healthcare provider or pharmacist if you are not sure if you take any of these medicines. Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I use Tegsedi?
- Read the detailed Instructions for Use that come with your Tegsedi.
- Your healthcare provider will show you or your caregiver how to inject Tegsedi the first time.
- If you or your caregiver have any questions, ask your healthcare provider.
- Tegsedi is injected under your skin (subcutaneously) in your stomach area (abdomen), or the front of your upper legs (thighs) by you or a caregiver. A caregiver may also give you an injection of Tegsedi in the outer area of your upper arm.
- Do not inject into the same site each time.
- Do not inject into the 2-inch area around the belly-button (naval).
- Do not inject where the skin is bruised, tender, red, or hard.
- Do not inject into areas with scars or tattoos.
- Do not inject through clothing.
- Follow your healthcare provider’s instructions on when to inject Tegsedi.
- Tegsedi should be injected 1 time each week on the same day.
- If you miss a dose, take the missed dose as soon as possible, unless your next scheduled dose is within 2 days. If your next scheduled dose is within 2 days, skip the missed dose and take your next scheduled dose on the scheduled day.
What are the possible side effects of Tegsedi?
Tegsedi may cause serious side effects, including:
- See “What is the most important information I should know about Tegsedi?”
- stroke. Tegsedi may cause a stroke. One person taking Tegsedi had a stroke, which occurred within 2 days after the first dose. Signs or symptoms of stroke may include:
- sudden numbness or weakness especially on one side of the body
- severe headache or neck pain
- confusion
- problems with vision, speech, or balance
- droopy eyelids
Get emergency help immediately if you have symptoms of stroke.
- inflammatory and immune system problems.Some people taking Tegsedi had serious inflammatory and immune system problems. Symptoms of inflammatory and immune system problems included unexpected change in walking, weakness and spasms in legs, back pain, weight loss, headache, vomiting, and problems with speech.
- liver effects. Tegsedi may cause liver problems. Your healthcare provider should do laboratory tests to check your liver before you start Tegsedi and while you are using it. Tell your healthcare provider if you have symptoms that your liver may not be working right, which could include unexpected nausea and vomiting, stomach pain, being not hungry, yellowing of the skin, or having dark urine.
- allergic reactions. Tegsedi may cause serious allergic reactions. These allergic reactions often occur within 2 hours after injecting Tegsedi. Get emergency help immediately if you have any symptoms of an allergic reaction including:
- joint pain
- chills
- redness on palms of hands
- muscle pain
- chest pain
- flushing
- tremor or jerking movements
- flu-like symptoms
- high blood pressure
- difficulty swallowing
- eye problems (low vitamin A levels). Treatment with Tegsedi will lower the Vitamin A levels in your blood. Your healthcare provider should tell you to take Vitamin A supplements while using Tegsedi. Your healthcare provider will tell you how much to take. Call your healthcare provider if you get eye problems, such as having difficulty seeing at night or in low lit areas (night blindness). Your healthcare provider should send you to see an eye doctor (ophthalmologist).
The most common side effects of Tegsedi include: injection site reactions (such as redness or pain at the injection site), nausea, headache, tiredness, low platelet counts (thrombocytopenia), and fever.
These are not all the possible side effects of Tegsedi.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tegsedi
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Tegsedi for a condition for which it has not been prescribed. Do not give Tegsedi to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Tegsedi that was written for health professionals.
How should I store Tegsedi?
- Store Tegsedi in the refrigerator between 36°F to 46°F (2°C to 8°C) in the original container.
- Do not freeze.
- Tegsedi prefilled syringes can also be kept at room temperature that is no higher than 86°F (30°C) in the original container for up to 6 weeks.
- Do not let Tegsedi reach temperatures above 86°F (30°C).
- If you do not use Tegsedi kept at room temperature within 6 weeks, throw it away.
- Protect from light.
Keep Tegsedi and all medicines out of the reach of children.
Label


What are the ingredients in Tegsedi?
Active ingredients: inotersen
Inactive ingredients: purified water (water for injection), hydrochloric acid and or sodium hydroxide for pH adjustment
For more information about Tegsedi, contact Akcea Therapeutics, Inc., at 1-844-483-4736 or go to www.TEGSEDIREMS.com.
SRC: NLM .
