Soliris
Generic name: eculizumab
Drug class: Selective immunosuppressants
Medically reviewed by A Ras MD.
What is Soliris?
Soliris is a prescription medicine called a monoclonal antibody. Soliris is used to treat patients with a disease called Paroxysmal Nocturnal Hemoglobinuria (PNH) adults and children with a disease called atypical Hemolytic Uremic Syndrome (aHUS)
Soliris is not for use in treating people with Shiga toxin E. coli related hemolytic uremic syndrome (STEC-HUS), adults with a disease called generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AchR) antibody positive adults with a disease called neuromyelitis optica spectrum disorder (NMOSD) who are anti-aquaporin-4 (AQP4) antibody positive.
It is not known if Soliris is safe and effective in children with PNH, gMG, or NMOSD.
Description
Eculizumab, a complement inhibitor, is a recombinant humanized monoclonal IgG2/4κ antibody produced by murine myeloma cell culture and purified by standard bioprocess technology. Eculizumab contains human constant regions from human IgG2 sequences and human IgG4 sequences and murine complementarity-determining regions grafted onto the human framework light- and heavy-chain variable regions. Eculizumab is composed of two 448 amino acid heavy chains and two 214 amino acid light chains and has a molecular weight of approximately 148 kDa.
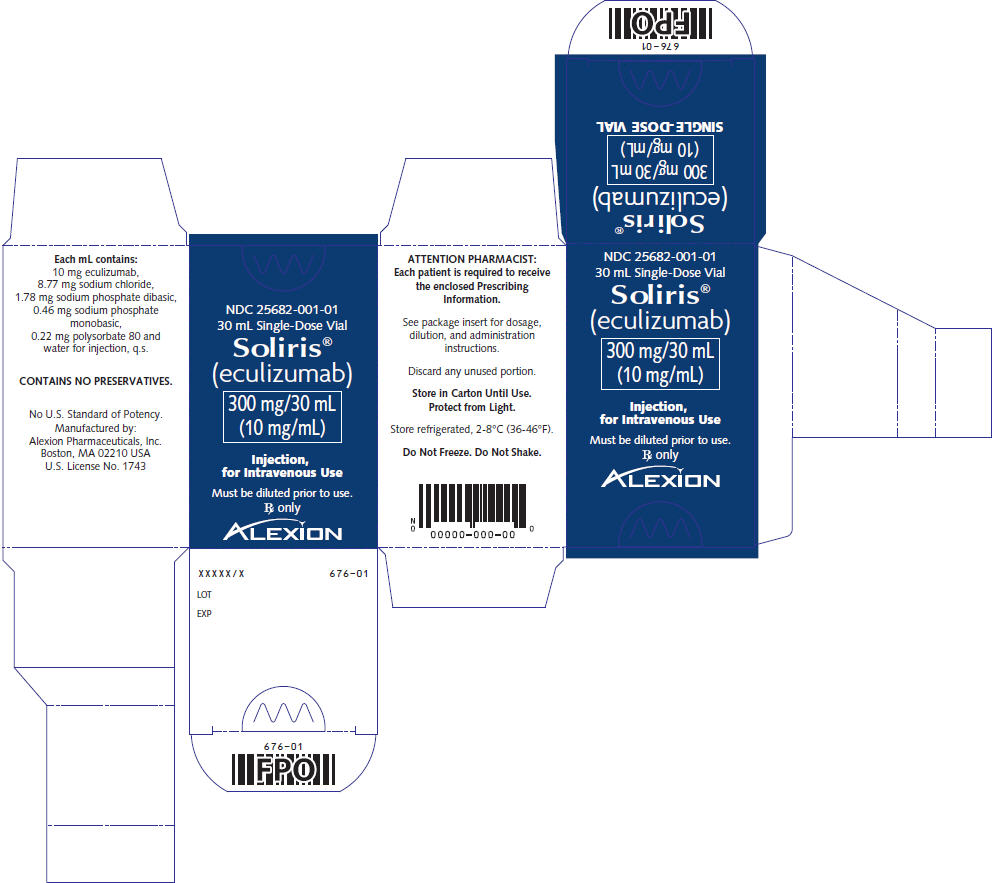
Soliris (eculizumab) injection is a sterile, clear, colorless, preservative-free 10 mg/mL solution for intravenous infusion and is supplied in 30-mL single-dose vials. The product is formulated at pH 7 and each 30 mL vial contains 300 mg of eculizumab, polysorbate 80 (6.6 mg) (vegetable origin), sodium chloride (263.1 mg), sodium phosphate dibasic (53.4 mg), sodium phosphate monobasic (13.8 mg), and Water for Injection, USP.
Mechanism of Action
Eculizumab, the active ingredient in Soliris, is a monoclonal antibody that specifically binds to the complement protein C5 with high affinity, thereby inhibiting its cleavage to C5a and C5b and preventing the generation of the terminal complement complex C5b-9.
Soliris inhibits terminal complement-mediated intravascular hemolysis in PNH patients and complement-mediated thrombotic microangiopathy (TMA) in patients with aHUS.
The precise mechanism by which eculizumab exerts its therapeutic effect in gMG patients is unknown, but is presumed to involve reduction of terminal complement complex C5b-9 deposition at the neuromuscular junction.
The precise mechanism by which eculizumab exerts its therapeutic effect in NMOSD is unknown, but is presumed to involve inhibition of aquaporin-4-antibody induced terminal complement C5b-9 deposition.
What is the most important information I should know about Soliris?
Soliris is a medicine that affects your immune system. Soliris can lower the ability of your immune system to fight infections.
- Soliris increases your chance of getting serious and life-threatening meningococcal infections. Meningococcal infections may quickly become life-threatening and cause death if not recognized and treated early.
1. You must receive meningococcal vaccines at least 2 weeks before your first dose of Soliris if you have not already had this vaccine.
2. If your doctor decided that urgent treatment with Soliris is needed, you should receive meningococcal vaccination as soon as possible.
3. If you have not been vaccinated and Soliris therapy must be initiated immediately, you should also receive two weeks of antibiotics with your vaccinations.
4. If you had a meningococcal vaccine in the past, you might need additional vaccination before starting Soliris. Your doctor will decide if you need additional meningococcal vaccination.
5. Meningococcal vaccines reduce the risk of meningococcal infection but do not prevent all meningococcal infections. Call your doctor or get emergency medical care right away if you get any of these signs and symptoms of a meningococcal infection:
- headache with nausea or vomiting
- headache with a stiff neck or stiff back
- fever and a rash
- muscle aches with flu-like symptoms
- headache and fever
- fever
- confusion
- eyes sensitive to light
Your doctor will give you a Patient Safety Card about the risk of meningococcal infection. Carry it with you at all times during treatment and for 3 months after your last Soliris dose. Your risk of meningococcal infection may continue for several weeks after your last dose of Soliris. It is important to show this card to any doctor or nurse who treats you. This will help them diagnose and treat you quickly.
Soliris is only available through a program called the Soliris REMS. Before you can receive Soliris, your doctor must:
- enroll in the Soliris REMS program
- counsel you about the risk of meningococcal infection
- give you information about the symptoms of meningococcal infection
- give you a Patient Safety Card about your risk of meningococcal infection, as discussed above
- make sure that you are vaccinated with the meningococcal vaccine and, if needed, get revaccinated with the meningococcal vaccine. Ask your doctor if you are not sure if you need to be revaccinated.
Soliris may also increase the risk of other types of serious infections. If your child is treated with Soliris, make sure that your child receives vaccinations against Streptococcus pneumoniae and Haemophilis influenza type b (Hib). Certain people may be at risk of serious infections with gonorrhea. Talk to your doctor about whether you are at risk for gonorrhea infection, about gonorrhea prevention, and regular testing. Certain fungal infections (aspergillus) may also happen if you take Soliris and have a weak immune system or a low white blood cell count.
Who should not use Soliris?
- have a meningococcal infection.
- have not been vaccinated against meningitis infection unless your doctor decides that urgent treatment with Soliris is needed. See “What is the most important information I should know about Soliris?”
What should I tell my healthcare provider before using Soliris?
Before you receive Soliris, tell your doctor about all of your medical conditions, including if you:
- have an infection or fever.
- are pregnant or plan to become pregnant. It is not known if Soliris will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Soliris passes into your breast milk.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Soliris and other medicines can affect each other causing side effects.
It is important that you:
- have all recommended vaccinations before you start Soliris.
- receive 2 weeks of antibiotics if you immediately start Soliris
- stay up-to-date with all recommended vaccinations during treatment with Soliris.
Know the medications you take and the vaccines you receive. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use Soliris?
- Soliris is given through a vein (I.V. or intravenous infusion) usually over 35 minutes in adults and 1 to 4 hours in pediatric patients. If you have an allergic reaction during your Soliris infusion, your doctor may decide to give Soliris more slowly or stop your infusion.
- If you are an adult, you will usually receive a Soliris infusion by your doctor:
- weekly for five weeks, then
- every 2 weeks
- If you are less than 18 years of age, your doctor will decide how often you will receive Soliris depending on your age and body weight
- After each infusion, you should be monitored for one hour for allergic reactions. See “What are the possible side effects of Soliris?”
- If you miss a Soliris infusion, call your doctor right away.
- If you have PNH, your doctor will need to monitor you closely for at least 8 weeks after stopping Soliris. Stopping treatment with Soliris may cause breakdown of your red blood cells due to PNH.
Symptoms or problems that can happen due to red blood cell breakdown include:- drop in the number of your red blood cell count
- kidney problems
- drop in your platelet counts
- blood clots
- confusion
- difficulty breathing
- chest pain
- If you have aHUS, your doctor will need to monitor you closely during and for at least 12 weeks after stopping treatment for signs of worsening aHUS symptoms or problems related to abnormal clotting (thrombotic microangiopathy).
Symptoms or problems that can happen with abnormal clotting may include:- stroke
- difficulty breathing
- confusion
- kidney problems
- seizure
- swelling in arms or legs
- chest pain (angina)
- a drop in your platelet count
What are the possible side effects of Soliris?
Soliris can cause serious side effects including:
- See “What is the most important information I should know about Soliris?”
- Serious allergic reactions. Serious allergic reactions can happen during your Soliris infusion. Tell your doctor or nurse right away if you get any of these symptoms during your Soliris infusion:If you have an allergic reaction to Soliris, your doctor may need to infuse Soliris more slowly, or stop Soliris. See “How should I take Soliris?”
- chest pain
- trouble breathing or shortness of breath
- swelling of your face, tongue, or throat
- feel faint or pass out
If you have an allergic reaction to Soliris, your doctor may need to infuse Soliris more slowly, or stop Soliris. See “How will I receive Soliris?”
The most common side effects in people with PNH treated with Soliris include:
- headache
- pain or swelling of your nose or throat (nasopharyngitis)
- back pain
- nausea
The most common side effects in people with aHUS treated with Soliris include:
- headache
- diarrhea
- high blood pressure (hypertension)
- common cold (upper respiratory infection
- stomach area (abdominal pain)
- vomiting
- pain or swelling of your nose or throat (nasopharyngitis)
- low red blood cell count (anemia)
- cough
- swelling of legs or feet (peripheral edema)
- nausea
- urinary tract infections
- fever
The most common side effects in people with gMG treated with Soliris include:
- muscle and joint (musculoskeletal) pain
The most common side effects in people with NMOSD treated with Soliris include:
- common cold (upper respiratory infection)
- pain or swelling of your nose or throat (nasopharyngitis)
- diarrhea
- back pain
- dizziness
- flu like symptoms (Influenza) including fever, headache, tiredness, cough, sore throat, and body aches
- joint pain (arthralgia)
- throat irritation (pharyngitis)
- bruising (contusion)
Tell your doctor about any side effect that bothers you or that does not go away. These are not all the possible side effects of SOLIRIS. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Soliris
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Soliris for a condition for which it was not prescribed. Do not give Soliris to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about Soliris that is written for health professionals.
What are the ingredients in Soliris?
Active ingredient: eculizumab
Inactive ingredients: polysorbate 80 (vegetable origin), sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic, and Water for Injection
Label
PRINCIPAL DISPLAY PANEL – 30 ML VIAL CARTON
- NDC 25682-001-01
30 mL Single-Dose Vial - Soliris®
(eculizumab) - 300 mg/30 mL
(10 mg/mL) - Injection,
for Intravenous Use - Must be diluted prior to use.
Rx only - ALEXION
SRC: NLM .
