Singulair
Generic name: montelukast sodium
What is Singulair?
Singulair is a prescription medicine that blocks substances in the body called leukotrienes. This may help to improve symptoms of asthma and allergic rhinitis. Singulair does not contain a steroid.
Singulair is used to:
1. Prevent asthma attacks and for the long-term treatment of asthma in adults and children ages 12 months and older.
Do not take Singulair if you need relief right away for a sudden asthma attack. If you get an asthma attack, you should follow the instructions your healthcare provider gave you for treating asthma attacks.
2. Prevent exercise-induced asthma in people 6 years of age and older.
3. Help control the symptoms of allergic rhinitis (sneezing, stuffy nose, runny nose, itching of the nose). Singulair is used to treat:
- outdoor allergies that happen part of the year (seasonal allergic rhinitis) in adults and children ages 2 years and older, and
- indoor allergies that happen all year (perennial allergic rhinitis) in adults and children ages 6 months and older.
Description
Montelukast sodium, the active ingredient in SINGULAIR, is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl leukotriene CysLT1 receptor.
Montelukast sodium is described chemically as [R-(E)]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic acid, monosodium salt.
The empirical formula is C35H35ClNNaO3S, and its molecular weight is 608.18. The structural formula is:
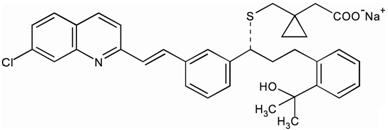 |
Montelukast sodium is a hygroscopic, optically active, white to off-white powder. Montelukast sodium is freely soluble in ethanol, methanol, and water and practically insoluble in acetonitrile.
Each 10-mg film-coated SINGULAIR tablet contains 10.4 mg montelukast sodium, which is equivalent to 10 mg of montelukast, and the following inactive ingredients: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The film coating consists of: hydroxypropyl methylcellulose, hydroxypropyl cellulose, titanium dioxide, red ferric oxide, yellow ferric oxide, and carnauba wax.
Each 4-mg and 5-mg chewable SINGULAIR tablet contains 4.2 and 5.2 mg montelukast sodium, respectively, which are equivalent to 4 and 5 mg of montelukast, respectively. Both chewable tablets contain the following inactive ingredients: mannitol, microcrystalline cellulose, hydroxypropyl cellulose, red ferric oxide, croscarmellose sodium, cherry flavor, aspartame, and magnesium stearate.
Each packet of SINGULAIR 4-mg oral granules contains 4.2 mg montelukast sodium, which is equivalent to 4 mg of montelukast. The oral granule formulation contains the following inactive ingredients: mannitol, hydroxypropyl cellulose, and magnesium stearate.
Mechanism of Action
The cysteinyl leukotrienes (LTC4, LTD4, LTE4) are products of arachidonic acid metabolism and are released from various cells, including mast cells and eosinophils. These eicosanoids bind to cysteinyl leukotriene (CysLT) receptors. The CysLT type-1 (CysLT1) receptor is found in the human airway (including airway smooth muscle cells and airway macrophages) and on other pro-inflammatory cells (including eosinophils and certain myeloid stem cells).
CysLTs have been correlated with the pathophysiology of asthma and allergic rhinitis. In asthma, leukotriene-mediated effects include airway edema, smooth muscle contraction, and altered cellular activity associated with the inflammatory process. In allergic rhinitis, CysLTs are released from the nasal mucosa after allergen exposure during both early- and late-phase reactions and are associated with symptoms of allergic rhinitis.
Montelukast is an orally active compound that binds with high affinity and selectivity to the CysLT1 receptor (in preference to other pharmacologically important airway receptors, such as the prostanoid, cholinergic, or β-adrenergic receptor). Montelukast inhibits physiologic actions of LTD4 at the CysLT1 receptor without any agonist activity.
Who should not take Singulair?
Do not take Singulair if you are allergic to any of its ingredients.
See below for a complete list of ingredients in Singulair.
What should I tell my healthcare provider before taking Singulair?
Before taking Singulair, tell your healthcare provider if you:
- are allergic to aspirin
- have phenylketonuria. Singulair chewable tablets contain aspartame, a source of phenylalanine
- have any other medical conditions
- are pregnant or plan to become pregnant. Talk to your doctor if you are pregnant or plan to become pregnant, as Singulair may not be right for you.
- are breast-feeding or plan to breast-feed. It is not known if Singulair passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while taking Singulair.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicines may affect how Singulair works, or Singulair may affect how your other medicines work.
How should I take Singulair?
For anyone who takes Singulair:
- Take Singulair exactly as prescribed by your healthcare provider. Your healthcare provider will tell you how much Singulair to take, and when to take it.
- Do not stop taking Singulair or change when you take it without talking with your healthcare provider.
- You can take Singulair with food or without food. See the information below in the section “How should I give Singulair oral granules to my child?” for information about what foods and liquids can be taken with Singulair oral granules.
- If you or your child misses a dose of Singulair, just take the next dose at your regular time. Do not take 2 doses at the same time.
- If you take too much Singulair, call your healthcare provider or a Poison Control Center right away.
For adults and children 12 months of age and older with asthma:
- Take Singulair 1 time each day, in the evening. Continue to take Singulair every day for as long as your healthcare provider prescribes it, even if you have no asthma symptoms.
- Tell your healthcare provider right away if your asthma symptoms get worse, or if you need to use your rescue inhaler medicine more often for asthma attacks.
- Do not take Singulair if you need relief right away from a sudden asthma attack. If you get an asthma attack, you should follow the instructions your healthcare provider gave you for treating asthma attacks.
- Always have your rescue inhaler medicine with you for asthma attacks.
- Do not stop taking or lower the dose of your other asthma medicines unless your healthcare provider tells you to.
For patients 6 years of age and older for the prevention of exercise-induced asthma:
- Take Singulair at least 2 hours before exercise.
- Always have your rescue inhaler medicine with you for asthma attacks.
- If you take Singulair every day for chronic asthma or allergic rhinitis, do not take another dose to prevent exercise-induced asthma. Talk to your healthcare provider about your treatment for exercise-induced asthma.
- Do not take 2 doses of Singulair within 24 hours (1 day).
For adults and children 2 years of age and older with seasonal allergic rhinitis, or for adults and children 6 months of age and older with perennial allergic rhinitis:
- Take Singulair 1 time each day, at about the same time each day.
How should I give Singulair oral granules to my child?
Give Singulair oral granules to your child exactly as instructed by your healthcare provider.
Do not open the packet until ready to use.
Singulair 4-mg oral granules can be given:
- right in the mouth; or
- dissolved in 1 teaspoonful (5 mL) of cold or room temperature baby formula or breast milk; or
- mixed with 1 spoonful of one of the following soft foods at cold or room temperature: applesauce, mashed carrots, rice, or ice cream.
Give the child all of the mixture right away, within 15 minutes.
Do not store any leftover Singulair mixture (oral granules mixed with food, baby formula, or breast milk) for use at a later time. Throw away any unused portion.
Do not mix Singulair oral granules with any liquid drink other than baby formula or breast milk. Your child may drink other liquids after swallowing the mixture.
What is the dose of Singulair?
The dose of Singulair prescribed for your or your child’s condition is based on age:
- 6 to 23 months: one packet of 4-mg oral granules.
- 2 to 5 years: one 4-mg chewable tablet or one packet of 4-mg oral granules.
- 6 to 14 years: one 5-mg chewable tablet.
- 15 years and older: one 10-mg tablet.
What should I avoid while taking Singulair?
If you have asthma and aspirin makes your asthma symptoms worse, continue to avoid taking aspirin or other medicines called non-steroidal anti-inflammatory drugs (NSAIDs) while taking Singulair.
What are the possible side effects of Singulair?
Singulair may cause serious side effects.
- Behavior and mood-related changes. Tell your healthcare provider right away if you or your child have any of these symptoms while taking Singulair:
- agitation including aggressive behavior or hostility
- attention problems
- bad or vivid dreams
- depression
- disorientation (confusion)
- feeling anxious
- hallucinations (seeing or hearing things that are not really there)
- irritability
- memory problems
- obsessive-compulsive symptoms
- restlessness
- sleep walking
- stuttering
- suicidal thoughts and actions (including suicide)
- tremor
- trouble sleeping
- uncontrolled muscle movements
- Increase in certain white blood cells (eosinophils) and possible inflamed blood vessels throughout the body (systemic vasculitis). Rarely, this can happen in people with asthma who take Singulair. This sometimes happens in people who also take a steroid medicine by mouth that is being stopped or the dose is being lowered. Tell your healthcare provider right away if you get one or more of these symptoms:
- a feeling of pins and needles or numbness of arms or legs
- a flu-like illness
- rash
- severe inflammation (pain and swelling) of the sinuses (sinusitis)
The most common side effects with Singulair include:
- upper respiratory infection
- fever
- headache
- sore throat
- cough
- stomach pain
- diarrhea
- earache or ear infection
- flu
- runny nose
- sinus infection
Other side effects with Singulair include:
- increased bleeding tendency, low blood platelet count
- allergic reactions [including swelling of the face, lips, tongue, and/or throat (which may cause trouble breathing or swallowing), hives and itching]
- dizziness, drowsiness, pins and needles/numbness, seizures (convulsions or fits)
- palpitations
- nose bleed, stuffy nose, swelling (inflammation) of the lungs
- heartburn, indigestion, inflammation of the pancreas, nausea, stomach or intestinal upset, vomiting
- hepatitis
- bruising, rash, severe skin reactions (erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis) that may occur without warning
- joint pain, muscle aches and muscle cramps
- bedwetting in children
- tiredness, swelling
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Singulair. For more information ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Lablel
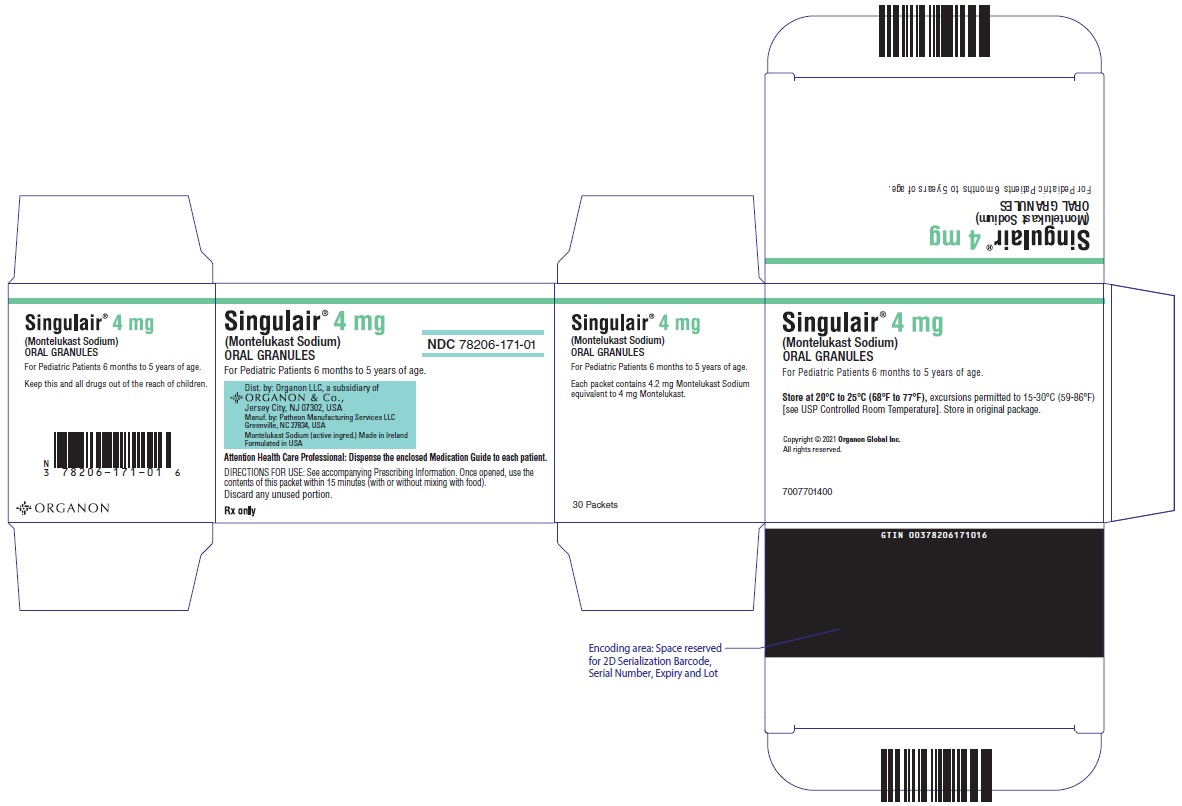
4 MG PACKET CARTON
- Singulair® 4 mg
(Montelukast Sodium)
ORAL GRANULES - NDC 78206-171-01
- For Pediatric Patients 6 months to 5 years of age.
- Dist. by: Organon LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA - Manuf. by: Patheon Manufacturing Services LLC
Greenville, NC 27834, USA - Montelukast Sodium (active ingred.) Made in Ireland
Formulated in USA - Attention Health Care Professional: Dispense the enclosed Medication Guide to each patient.
- DIRECTIONS FOR USE: See accompanying Prescribing Information. Once opened, use the
contents of this packet within 15 minutes (with or without mixing with food).
Discard any unused portion. - Rx only
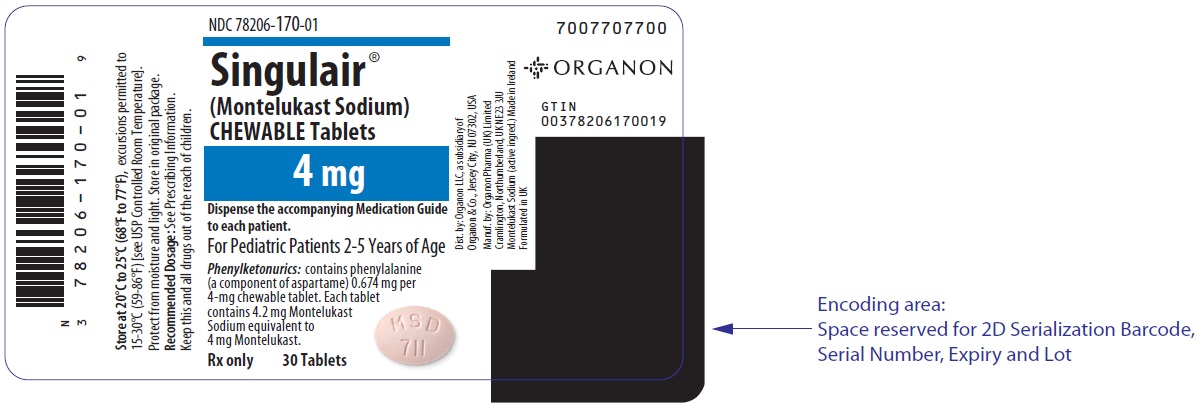
4 MG TABLET BOTTLE LABEL
- NDC 78206-170-01
- Singulair®
(Montelukast Sodium)
CHEWABLE Tablets - 4 mg
- Dispense the accompanying Medication Guide
to each patient. - For Pediatric Patients 2-5 Years of Age
- Phenylketonurics: contains phenylalanine
(a component of aspartame) 0.674 mg per
4-mg chewable tablet. Each tablet
contains 4.2 mg Montelukast
Sodium equivalent to
4 mg Montelukast. - Rx only 30 Tablets

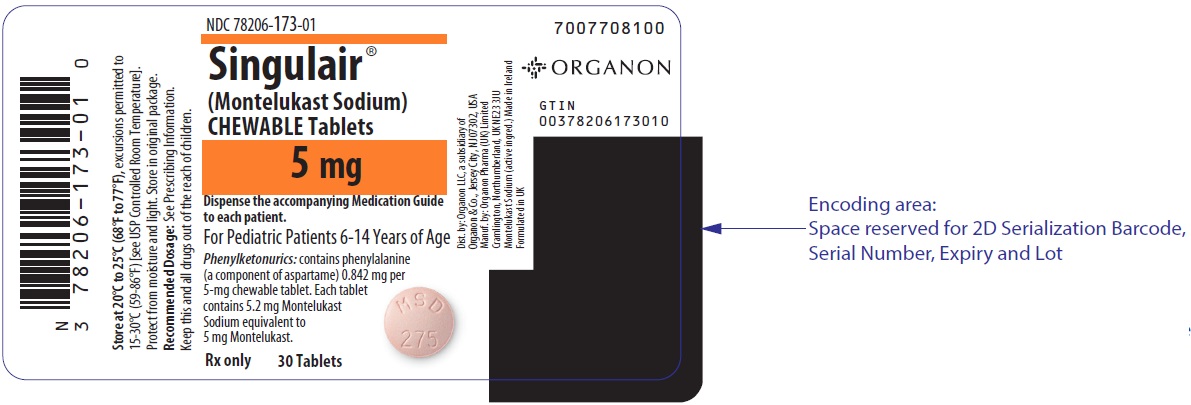
5 MG TABLET BOTTLE LABEL
- NDC 78206-173-01
- Singulair®
(Montelukast Sodium)
CHEWABLE Tablets - 5 mg
- Dispense the accompanying Medication Guide
to each patient. - For Pediatric Patients 6-14 Years of Age
- Phenylketonurics: contains phenylalanine
(a component of aspartame) 0.842 mg per
5-mg chewable tablet. Each tablet
contains 5.2 mg Montelukast
Sodium equivalent to
5 mg Montelukast. - Rx only 30 Tablets

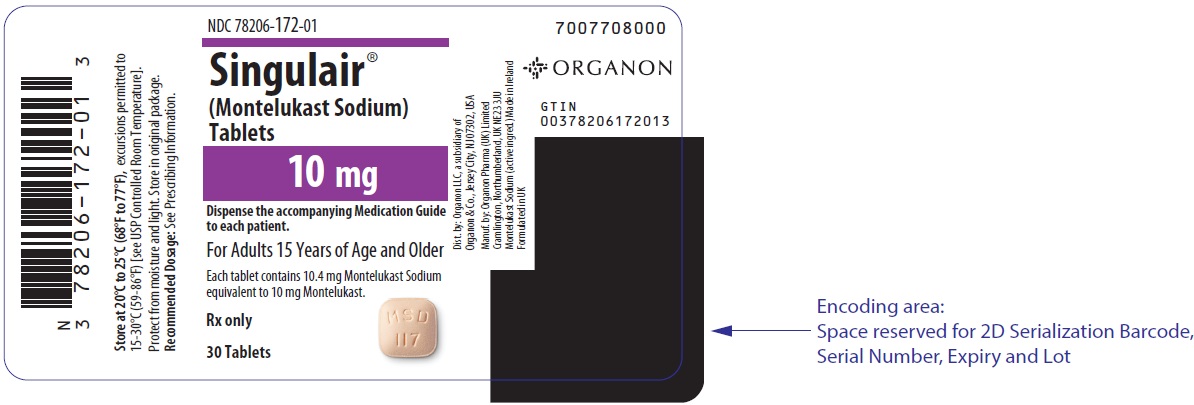
10 MG TABLET BOTTLE LABEL
- NDC 78206-172-01
- Singulair®
(Montelukast Sodium)
Tablets - 10 mg
- Dispense the accompanying Medication Guide
to each patient. - For Adults 15 Years of Age and Older
- Each tablet contains 10.4 mg Montelukast Sodium
equivalent to 10 mg Montelukast. - Rx only
- 30 Tablets
Drug Interactions
A total of 88 medications are known to interact with Singulair. Use the Interactions Checker Tool.
Common Interactions Checks
- Advair Diskus
- albuterol
- Cymbalta
- Flonase
- gabapentin
- lisinopril
- metformin
- omeprazole
- prednisone
- Synthroid
- Vitamin D3
- Zyrtec
General Information about the safe and effective use of Singulair
Medicines are sometimes prescribed for purposes other than those mentioned in Patient Information Leaflets. Do not use Singulair for a condition for which it was not prescribed. Do not give Singulair to other people even if they have the same symptoms you have. It may harm them. Keep Singulair and all medicines out of the reach of children.
This guide summarizes information about Singulair. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about Singulair that is written for health professionals. For more information, call the Merck National Service Center at 1-800-NSC-Merck (1-800-672-6372).
How should I store Singulair?
- Store Singulair at 59°F to 86°F (15°C to 30°C).
- Keep Singulair in the container it comes in.
- Keep Singulair in a dry place and away from light.
What are the ingredients in Singulair?
Active ingredient: montelukast sodium
Inactive ingredients:
Granules: mannitol, hydroxypropyl cellulose, and magnesium stearate.
Chewable tablets: mannitol, microcrystalline cellulose, hydroxypropyl cellulose, red ferric oxide, croscarmellose sodium, cherry flavor, aspartame, and magnesium stearate.
- People with Phenylketonuria: Singulair 4-mg chewable tablets contain 0.674 mg of phenylalanine, and Singulair 5-mg chewable tablets contain 0.842 mg of phenylalanine.
Tablet: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The film coating contains: hydroxypropyl methylcellulose, hydroxypropyl cellulose, titanium dioxide, red ferr
SRC: NLM .
