Proair HFA
Generic name: albuterol inhalation
Brand names: ProAir HFA, ProAir RespiClick, Proventil HFA, Ventolin HFA
Drug class: Adrenergic bronchodilators
Medically reviewed by A Ras MD.
What is Proair HFA?
Proair HFA is a prescription medicine used in people 4 years of age and older to treat or prevent bronchospasm in people who have reversible obstructive airway disease, and to prevent exercise induced bronchospasm
It is not known if Proair HFA is safe and effective in children under 4 years of age.
Description
The active ingredient of PROAIR HFA (albuterol sulfate) Inhalation Aerosol is albuterol sulfate, a racemic salt, of albuterol. Albuterol sulfate has the chemical name α1-[(tert-butylamino) methyl]-4-hydroxy-m-xylene-α,α’-diol sulfate (2:1) (salt), and has the following chemical structure:
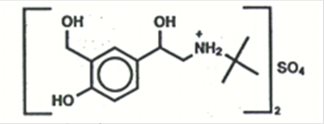
The molecular weight of albuterol sulfate is 576.7, and the empirical formula is (C13H21NO3)2•H2SO4. Albuterol sulfate is a white to off-white crystalline powder. It is soluble in water and slightly soluble in ethanol. Albuterol sulfate is the official generic name in the United States, and salbutamol sulfate is the World Health Organization recommended generic name. PROAIR HFA Inhalation Aerosol is a pressurized metered-dose aerosol unit with a dose counter. PROAIR HFA is for oral inhalation only. It contains a microcrystalline suspension of albuterol sulfate in propellant HFA-134a (1, 1, 1, 2-tetrafluoroethane) and ethanol.
Prime the inhaler before using for the first time and in cases where the inhaler has not been used for more than 2 weeks by releasing three sprays into the air, away from the face. After priming, each actuation delivers 108 mcg albuterol sulfate, from the actuator mouthpiece (equivalent to 90 mcg of albuterol base). Each canister provides 200 actuations (inhalations).
This product does not contain chlorofluorocarbons (CFCs) as the propellant.
Mechanism of Action
Albuterol sulfate is a beta2-adrenergic agonist. The pharmacologic effects of albuterol sulfate are attributable to activation of beta2-adrenergic receptors on airway smooth muscle. Activation of beta2-adrenergic receptors leads to the activation of adenylcyclase and to an increase in the intracellular concentration of cyclic-3′, 5′-adenosine monophosphate (cyclic AMP). This increase of cyclic AMP is associated with the activation of protein kinase A, which in turn inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in muscle relaxation. Albuterol relaxes the smooth muscle of all airways, from the trachea to the terminal bronchioles. Albuterol acts as a functional antagonist to relax the airway irrespective of the spasmogen involved, thus protecting against all bronchoconstrictor challenges. Increased cyclic AMP concentrations are also associated with the inhibition of release of mediators from mast cells in the airway. While it is recognized that beta2-adrenergic receptors are the predominant receptors on bronchial smooth muscle, data indicate that there are beta-receptors in the human heart, 10% to 50% of which are cardiac beta2-adrenergic receptors. The precise function of these receptors has not been established .
Albuterol has been shown in most controlled clinical trials to have more effect on the respiratory tract, in the form of bronchial smooth muscle relaxation, than isoproterenol at comparable doses while producing fewer cardiovascular effects. However, inhaled albuterol, like other beta-adrenergic agonist drugs, can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes .
Who should not use Proair HFA?
Do not use Proair HFA if you are allergic to albuterol sulfate or any of the ingredients in Proair HFA. See the end of this leaflet for a complete list of ingredients in Proair HFA.
What should I tell my healthcare provider before I use Proair HFA?
Before you use Proair HFA, tell your doctor if you:
- have heart problems
- have high blood pressure (hypertension)
- have convulsions (seizures)
- have thyroid problems
- have diabetes
- have low potassium levels in your blood
- are pregnant or plan to become pregnant. It is not known if Proair HFA will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if Proair HFA passes into your breast milk. Talk to your doctor about the best way to feed your baby if you are using Proair HFA.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Proair HFA and other medicines may affect each other and cause side effects. Proair HFA may affect the way other medicines work, and other medicines may affect the way Proair HFA works.
Especially tell your doctor if you take:
- other inhaled medicines or asthma medicines
- beta blocker medicines
- diuretics
- digoxin
- monoamine oxidase inhibitors
- tricyclic antidepressants
Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use Proair HFA?
- For detailed instructions, see the instructions for use at the end of this Patient Information.
- Use Proair HFA exactly as your doctor tells you to use it.
- If your child needs to use Proair HFA, watch your child closely to make sure your child uses the inhaler correctly. Your doctor will show you how your child should use Proair HFA.
- Each dose of Proair HFA should last up to 4 hours to 6 hours.
- Do not increase your dose or take extra doses of Proair HFA without first talking to your doctor.
- Get medical help right away if Proair HFA no longer helps your symptoms.
- Get medical help right away if your symptoms get worse or if you need to use your inhaler more often.
- While you are using Proair HFA, do not use other inhaled rescue medicines and asthma medicines unless your doctor tells you to do so.
- Call your doctor if your asthma symptoms like wheezing and trouble breathing become worse over a few hours or days. Your doctor may need to give you another medicine (for example, corticosteroids) to treat your symptoms.
What are the possible side effects of Proair HFA?
Proair HFA may cause serious side effects, including:
- worsening trouble breathing, coughing and wheezing (paradoxical bronchospasm). If this happens stop using Proair HFA and call your doctor or get emergency help right away. Paradoxical bronchospasm is more likely to happen with your first use of a new canister of medicine.
- heart problems including faster heart rate and higher blood pressure
- possible death in people with asthma who use too much Proair HFA
- allergic reactions. Call your doctor right away if you have the following symptoms of an allergic reaction:
- itchy skin
- swelling beneath your skin or in your throat
- rash
- worsening trouble breathing
- low potassium levels in your blood
- worsening of other medical problems in people who also use Proair HFA including increases in blood sugar
The most common side effects of Proair HFA include:
- your heart feels like it is pounding or racing (palpitations)
- chest pain
- fast heart rate
- shakiness
- nervousness
- headache
- dizziness
- sore throat
- runny nose
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of Proair HFA. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Drug Interactions
A total of 405 medications are known to interact with Proair HFA. Use the Interactions Checker Tool.
Common Interactions Checks
- Advair Diskus
- atorvastatin
- gabapentin
- levothyroxine
- lisinopril
- metformin
- montelukast
- omeprazole
- prednisone
- Singulair
- Symbicort
- Vitamin D3
General Information about the safe and effective use of Proair HFA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Proair HFA for a condition for which it was not prescribed. Do not give Proair HFA to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information summarizes the most important information about Proair HFA. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Proair HFA that is written for health professionals.
For more information, go to www.ProairHFA.com or call 1-888-483-8279.
How should I store Proair HFA?
- Store Proair HFA at room temperature between 59° F and 77° F (15° C and 25° C).
- Avoid exposure to extreme heat and cold.
- Shake the Proair HFA canister well before use.
- Do not puncture the Proair HFA canister.
- Do not store the Proair HFA canister near heat or a flame. Temperatures above 120° F may cause the canister to burst.
- Do not throw the Proair HFA canister into a fire or an incinerator.
- Avoid spraying Proair HFA in your eyes.
Keep Proair HFA and all medicines out of the reach of children.
What are the ingredients in Proair HFA?
Active ingredient: albuterol sulfate
Inactive ingredients: propellant HFA-134a and ethanol.
Instructions for use for ProAir HFA
ProAir HFA (prō’ ār)
(albuterol sulfate)
Inhalation Aerosol
The Parts of Your ProAir HFA Inhaler Device:
There are 2 main parts of your ProAir HFA inhaler device including a:
- red plastic actuator that sprays the medicine from the canister. See Figure A.
- protective dust cap that covers the mouthpiece of the actuator. See Figure A.
There is also a metal canister that holds the medicine. See Figure A.
There is also a dose counter attached to the back of the actuator with a viewing window that shows you how many sprays of medicine you have left. See Figure B.
You will see a black dot in the viewing window on the actuator until the device has been primed 3 times. See Figure B and “Priming Your ProAir HFA Device” below.
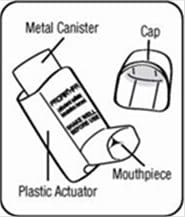
Figure A
Figure B
- Do not use the ProAir HFA actuator with a canister of medicine from any other inhaler.
- Do not use a ProAir HFA canister with an actuator from any other inhaler, including another ProAir HFA inhaler.
Priming Your ProAir HFA Device:
Your ProAir device must be primed before you use it for the first time or if your device has not been used for more than 14 days in a row. Do not prime your ProAir HFA device every day.
- Remove your ProAir HFA device from its package.
- Remove the protective dust cap from the mouthpiece.
- Shake the inhaler well, and spray it into the air away from your face. See Figure C.
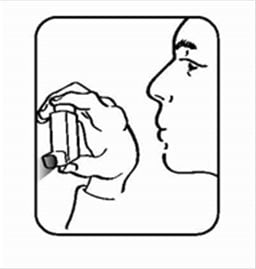
Figure C
- Shake and spray the inhaler like this 2 more times to finish priming it.
The dose counter on the actuator should display the number 200 after you prime the actuator for the first time. See Figure D.
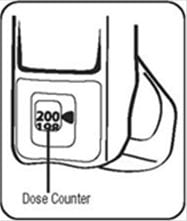
Figure D
Each Time You Use Your ProAir HFA Device:
- Make sure the canister fits firmly in the plastic actuator.
- Look into the mouthpiece to make sure there are no foreign objects there, especially if the cap has not been used to cover the mouthpiece.
Reading the Dose Counter on Your ProAir HFA Actuator
- The dose counter will count down each time a spray is released. The dose counter window shows the number of sprays left in your inhaler in units of 2 sprays. For example, there are 190 sprays left if the arrow is exactly opposite the number 190, or 189 sprays left if the arrow points between 190 and 188. See Figure D.
- When the dose counter reaches 0, it will continue to show 0 and you should replace your ProAir HFA device.
- The dose counter cannot be reset and is permanently attached to the actuator. Never change the numbers for the dose counter or touch the pin inside the actuator.
- Do not remove the canister from the plastic actuator except during cleaning. Reattaching the canister to the actuator may accidentally release a dose of ProAir HFA into the air. The dose counter will count down each time a spray is released.
Using Your ProAir HFA Device:
Step 1. Shake the inhaler well before each spray. Take the cap off the mouthpiece of the actuator.
Step 2. Hold the inhaler with the mouthpiece down. See Figure E.
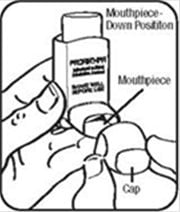
Figure E
Step 3. Breathe out through your mouth and push as much air from your lungs as you can. Put the mouthpiece in your mouth and close your lips around it. See Figure F.
Step 4. Push the top of the canister all the way down while you breathe in deeply and slowly through your mouth. See Figure F.

Figure F
Step 5. Right after the spray comes out, take your finger off the canister. After you have breathed in all the way, take the inhaler out of your mouth and close your mouth.
Step 6. Hold your breath as long as you can, up to 10 seconds, then breathe normally.
If your doctor has told you to use more sprays, wait 1 minute and shake the inhaler again. Repeat Steps 2 through Step 6.
Step 7. Put the cap back on the mouthpiece after every time you use the inhaler. Make sure the cap snaps firmly into place.
Cleaning Your ProAir HFA Device:
It is very important to keep the plastic actuator clean so the medicine will not build-up and block the spray. See Figure G and Figure H.
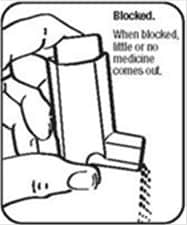
Figure G
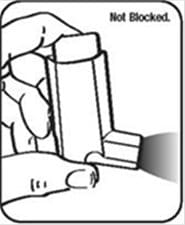
Figure H
- Do not try to clean the metal canister or let it get wet. The inhaler may stop spraying if it is not cleaned correctly.
- If you have more than 1 ProAir HFA inhaler, wash each device at separate times to prevent putting the wrong canister together with the wrong plastic actuator. This way you can be sure you will always know the correct number of remaining doses of ProAir HFA.
- Wash the actuator at least 1 time each week as follows:
- Take the canister out of the actuator, and take the cap off the mouthpiece.
- Hold the actuator under the faucet and run warm water through it for about 30 seconds. See Figure I.
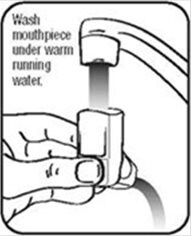
Figure I - Turn the actuator upside down and run warm water through the mouthpiece for about 30 seconds. See Figure J.
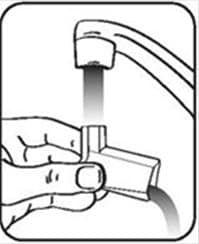
Figure J - Shake off as much water from the actuator as you can. Look into the mouthpiece to make sure any medicine build-up has been completely washed away. If there is any build-up, repeat the washing instructions.
- Let the actuator air-dry completely, such as overnight. See Figure K.
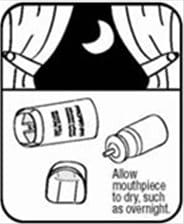
Figure K - When the actuator is dry, put the canister in the actuator and make sure it fits firmly. Shake the inhaler well and spray it twice into the air away from your face. Put the cap back on the mouthpiece.
If you need to use your inhaler before the actuator is completely dry:
- Shake as much water off the actuator as you can.
- Put the canister in the actuator and make sure it fits firmly.
- Shake the inhaler well and spray it twice into the air away from your face.
- Take your ProAir HFA dose as prescribed.
- Follow the Cleaning Instructions above.
Replacing Your ProAir HFA Device
- When the dose counter on the actuator says the number 20, the color of the numbers will change to red. The red numbers are to remind you to refill your prescription or ask your doctor for another prescription for ProAir HFA. When the dose counter reaches 0, the background color will change to solid red.
- Throw the ProAir HFA inhaler away as soon as the dose counter says 0 or after the expiration date on the ProAir HFA packaging, whichever comes first. You should not keep using the inhaler after 200 sprays even though the canister may not be completely empty. You cannot be sure you will receive any medicine after using 200 sprays.
- Do not use the inhaler after the expiration date on the ProAir HFA packaging.
Label
- ALBUTEROL SULFATE

