Nexlizet
Generic name: bempedoic acid and ezetimibe (tablets)
Drug class: Antihyperlipidemic combinations
Medically reviewed by A Ras MD.
What is Nexlizet?
Nexlizet is a prescription medicine that contains 2 cholesterol lowering medicines, bempedoic acid and ezetimibe. Nexlizet is used along with diet and other lipid-lowering medicines in the treatment of adults with:
- heterozygous familial hypercholesterolemia (HeFH). HeFH is an inherited condition that causes high levels of “bad” cholesterol called low density lipoprotein (LDL).
- known heart disease who need additional lowering of “bad” cholesterol (LDL-C) levels.
It is not known if Nexlizet can decrease problems from high cholesterol, such as heart attacks, stroke, death, or other heart problems.
It is not known if Nexlizet is safe and effective in people with severe kidney problems including people with end-stage kidney disease who are on dialysis.
It is not known if Nexlizet is safe and effective in people with moderate to severe liver problems.
It is not known if Nexlizet is safe and effective in children under 18 years of age.
Description
Nexlizettablets, for oral use, contain bempedoic acid, an adenosine triphosphate-citrate lyase (ACL) inhibitor, and ezetimibe, a dietary cholesterol absorption inhibitor.
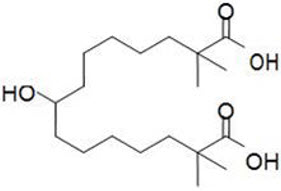
The chemical name for bempedoic acid is 8-hydroxy-2,2,14,14-tetramethyl-pentadecanedioic acid. The molecular formula is C19H36O5, and the molecular weight is 344.5 grams per mole. Bempedoic acid is a white to off-white crystalline powder that is highly soluble in ethanol, isopropanol and pH 8.0 phosphate buffer, and insoluble in water and aqueous solutions below pH 5.
Structural formula:
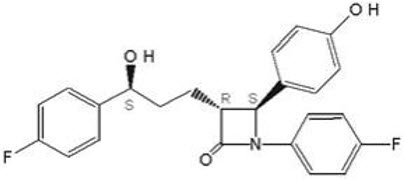
The chemical name for ezetimibe is 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone. The molecular formula is C24H21F2NO3 and the molecular weight is 409.4 grams per mole. Ezetimibe is a white, crystalline powder that is freely to very soluble in ethanol, methanol, and acetone and practically insoluble in water.
Structural formula:
Each film-coated tablet of NEXLIZET contains 180 mg of bempedoic acid and 10 mg of ezetimibe, and the following inactive ingredients: colloidal silicon dioxide, hydroxy propyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone K30, sodium lauryl sulfate, sodium starch glycolate. The film coating comprises of FD&C Blue #1/Brilliant Blue FCF Aluminum Lake, FD&C Blue #2/Indigo Carmine Aluminum Lake, glyceryl monocaprylocaprate, partially hydrolyzed polyvinyl alcohol, sodium lauryl sulfate, talc, and titanium dioxide.
Mechanism of Action
Nexlizet contains bempedoic acid and ezetimibe. NEXLIZET reduces elevated LDL-C through inhibition of cholesterol synthesis in the liver and absorption in the intestine.
Bempedoic acid
Bempedoic acid is an adenosine triphosphate-citrate lyase (ACL) inhibitor that lowers low-density lipoprotein cholesterol (LDL-C) by inhibition of cholesterol synthesis in the liver. ACL is an enzyme upstream of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase in the cholesterol biosynthesis pathway. Bempedoic acid and its active metabolite, ESP15228, require coenzyme A (CoA) activation by very long-chain acyl-CoA synthetase 1 (ACSVL1) to ETC-1002-CoA and ESP15228-CoA, respectively. ACSVL1 is expressed primarily in the liver. Inhibition of ACL by ETC-1002-CoA results in decreased cholesterol synthesis in the liver and lowers LDL-C in blood via upregulation of low-density lipoprotein receptors.
Ezetimibe
Ezetimibe reduces blood cholesterol by inhibiting the absorption of cholesterol by the small intestine. The molecular target of ezetimibe has been shown to be the sterol transporter, Niemann-Pick C1-Like 1 (NPC1L1), which is involved in the intestinal uptake of cholesterol and phytosterols. Ezetimibe localizes at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic choleste
Who should not take Nexlizet?
Do not take Nexlizet if you are allergic to ezetimibe tablets. Ezetimibe, one of the active ingredients in Nexlizet, can cause serious allergic reactions such as anaphylaxis, angioedema, rash, and urticaria. Stop taking Nexlizet, call your healthcare provider or go to the nearest hospital emergency room right away if you have any signs or symptoms of an allergic reaction including:
- swelling of your face, lips, mouth or tongue
- wheezing
- severe itching
- fast heart beat or pounding in your chest
- trouble breathing
- skin rashes, redness, or swelling
- dizziness or fainting
See the end of this guide for a complete list of ingredients in Nexlizet.
What should I tell my healthcare provider before taking Nexlizet?
Before you start taking Nexlizet, tell your healthcare provider about all your medical conditions, including if you:
- have or had gout.
- have or had tendon problems.
- are pregnant or think you may be pregnant. Tell your healthcare provider right away if you become pregnant while taking Nexlizet. You and your healthcare provider will decide if you should take Nexlizet while you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if Nexlizet passes into your breast milk. You and your healthcare provider should decide if you will take Nexlizet or breastfeed. You should not do both.
- have severe kidney problems.
- have moderate or severe liver problems.
Nexlizet may affect the way other medicines work, and other medicines may affect how Nexlizet works. Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take or plan to take:
- simvastatin or pravastatin (other cholesterol lowering medicines). Taking simvastatin or pravastatin with Nexlizet may increase your risk of developing muscle pain or weakness (myopathy).
- cyclosporine (often used in organ transplant patients)
- fibrates (used to lower cholesterol)
- cholestyramine (used to lower cholesterol)
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Nexlizet?
- Take Nexlizet exactly as your healthcare provider tells you to take it. Check with your healthcare provider or pharmacist if you are not sure.
- Take 1 Nexlizet tablet by mouth each day.
- Swallow the Nexlizet tablet whole. Do not cut, chew, or crush the tablet.
- You may take Nexlizet with or without food.
- If you take a medicine that lowers cholesterol by binding bile acids, such as colesevelam or cholestyramine, take Nexlizet at least 2 hours before or 4 hours after you take bile acid binding medicines. Ask your healthcare provider if you are not sure if you take these medicines.
- If you take too much Nexlizet, call your poison control center at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What are the possible side effects of Nexlizet?
Nexlizet may cause serious side effects, including:
- increased levels of uric acid in your blood (hyperuricemia). This can happen within 4 weeks of you starting Nexlizet and continue throughout your treatment. Your healthcare provider may monitor your blood uric acid levels while you are taking Nexlizet. High levels of blood uric acid may lead to gout. Call your healthcare provider if you have the following symptoms of hyperuricemia and gout:
- severe foot pain especially in the toe joint
- warm joints
- swelling
- tender joints
- joint rednessGout may happen more in people who have had gout before but also can happen in people who have never had it before.
- tendon rupture or injury. Tendon problems can happen in people who take bempedoic acid, one of the medicines in Nexlizet. Tendons are tough cords of tissue that connect muscles to bones. Symptoms of tendon problems may include pain, swelling, tears, and inflammation of tendons including the arm, shoulder, and back of the ankle (Achilles).
- Tendon rupture can happen while you are taking Nexlizet. Tendon ruptures can happen within weeks or months of starting Nexlizet.
- The risk of getting tendon problems while you take Nexlizet is higher if you:
- are over 60 years of age
- are taking antibiotics (fluoroquinolones)
- have had tendon problems
- are taking steroids (corticosteroids)
- have renal failure
- Stop taking Nexlizet immediately and get medical help right away if you get any of the following signs or symptoms of a tendon rupture:
- hear or feel a snap or pop in a tendon area
- bruising right after an injury in a tendon area
- unable to move the affected area or put weight on the affected areaStop taking Nexlizet until tendon rupture has been ruled out by your healthcare provider. Avoid exercise and using the affected area. The most common areas of pain and swelling are the rotator cuff (the shoulder), the biceps tendon (upper arm), and the Achilles tendon at the back of the ankle. This can happen with other tendons.
- Talk to your healthcare provider about the risk of tendon rupture with continued use of Nexlizet. You may need a different lipid-lowering medicine to treat your cholesterol levels.
The most common side effects of Nexlizet include:
- symptoms of the common cold, flu, or flu-like symptoms
- back pain
- bronchitis
- anemia
- diarrhea
- muscle spasms
- stomach pain
- pain in shoulder, legs, or arms
- increased liver enzymes
- fatigue
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Nexlizet. Ask your healthcare provider or pharmacist for more information.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Label
PRINCIPAL DISPLAY PANEL – 180 MG/10 MG TABLET BOTTLE LABEL
- NDC 72426-818-03
Rx only - NEXLIZET™
(bempedoic acid
and ezetimibe) tablets - Contains
30 Tablets - 180 mg/10 mg
General information about the safe and effective use of Nexlizet
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Nexlizet for a condition for which it was not prescribed. Do not give Nexlizet to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about Nexlizet that is written for healthcare professionals.
How should I store Nexlizet?
- Store Nexlizet in the original package at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- Protect from heat and moisture.
- Do not throw away the packet that helps to keep your medicine dry (desiccant).
- Nexlizet comes in a bottle with a child-resistant cap.
Keep Nexlizet and all medicines out of the reach of children.
What are the ingredients in Nexlizet?
- active ingredients: bempedoic acid and ezetimibe
- inactive ingredients: colloidal silicon dioxide, hydroxy propyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone K30, sodium lauryl sulfate, sodium starch glycolate
- tablet coating: FD&C Blue #1/Brilliant Blue FCF Aluminum Lake, FD&C Blue #2/Indigo Carmine Aluminum Lake, glyceryl monocaprylocaprate, partially hydrolyzed polyvinyl alcohol, sodium lauryl sulfate, talc, and titanium dioxide
SRC: NLM .
