Micardis HCT
Generic name: hydrochlorothiazide and telmisartan
Drug class: Angiotensin II inhibitors with thiazides
Medically reviewed by A Ras MD.
What is Micardis HCT?
Micardis HCT is a prescription medicine used to treat high blood pressure (hypertension). Micardis HCT contains telmisartan, an angiotensin receptor blocker (ARB), hydrochlorothiazide, a water pill or diuretic
Your doctor may prescribe other medicines for you to take along with Micardis HCT to treat your high blood pressure.
It is not known if Micardis HCT is safe and effective in children.
What is high blood pressure (hypertension)?
Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. Medicines that lower your blood pressure lower your chance of having a stroke or heart attack.
High blood pressure makes the heart work harder to pump blood through the body and causes damage to the blood vessels. Micardis HCT tablets can help your blood vessels relax so your blood pressure is lower.
Description
MICARDIS HCT tablets are a combination of telmisartan, an orally active angiotensin II antagonist acting on the AT1 receptor subtype, and hydrochlorothiazide, a thiazide diuretic.
Telmisartan, a non-peptide molecule, is chemically described as 4′-[(1,4′-dimethyl-2′-propyl[2,6′-bi-1H-benzimidazol]-1′-yl)methyl]-[1,1′-biphenyl]-2-carboxylic acid. Its empirical formula is C33H30N4O2, its molecular weight is 514.63, and its structural formula is:
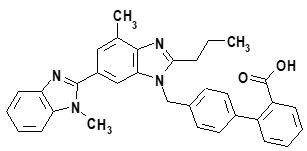
Telmisartan is a white to slightly yellowish solid. It is practically insoluble in water and in the pH range of 3 to 9, sparingly soluble in strong acid (except insoluble in hydrochloric acid), and soluble in strong base.
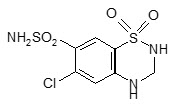
Hydrochlorothiazide is a white, or practically white, practically odorless, crystalline powder with a molecular weight of 297.74. It is slightly soluble in water, and freely soluble in sodium hydroxide solution. Hydrochlorothiazide is chemically described as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2, and its structural formula is:
MICARDIS HCT tablets are formulated for oral administration in three combinations of 40 mg/12.5 mg, 80 mg/12.5 mg, and 80 mg/25 mg telmisartan and hydrochlorothiazide, respectively. The tablets contain the following inactive ingredients: sodium hydroxide, meglumine, povidone, sorbitol, magnesium stearate, lactose monohydrate, microcrystalline cellulose, maize starch, and sodium starch glycolate. As coloring agents, the 40 mg/12.5 mg and 80 mg/12.5 mg tablets contain ferric oxide red, and the 80 mg/25 mg tablets contain ferric oxide yellow. MICARDIS HCT tablets are hygroscopic and require protection from moisture.
Mechanism of Action
MICARDIS HCT
MICARDIS HCT is a combination of two drugs with antihypertensive properties: a thiazide diuretic, hydrochlorothiazide, and an angiotensin II receptor blocker (ARB), telmisartan.
Telmisartan
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Telmisartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is therefore independent of the pathways for angiotensin II synthesis.
There is also an AT2 receptor found in many tissues, but AT2 is not known to be associated with cardiovascular homeostasis. Telmisartan has much greater affinity (>3,000-fold) for the AT1 receptor than for the AT2 receptor.
Telmisartan does not inhibit ACE (kininase II) nor does it bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of telmisartan on blood pressure.
Hydrochlorothiazide
Hydrochlorothiazide is a thiazide diuretic. Thiazides affect the renal tubular mechanisms of electrolyte reabsorption, directly increasing excretion of sodium salt and chloride in approximately equivalent amounts. Indirectly, the diuretic action of hydrochlorothiazide reduces plasma volume, with consequent increases in plasma renin activity, increases in aldosterone secretion, increases in urinary potassium loss, and decreases in serum potassium. The renin-aldosterone link is mediated by angiotensin II, so co-administration of an ARB tends to reverse the potassium loss associated with these diuretics. The mechanism of the antihypertensive effect of thiazides is not fully understood.
What is the most important information I should know about Micardis HCT?
Micardis HCT can cause harm or death to an unborn baby. Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant. If you get pregnant while taking Micardis HCT, tell your doctor right away.
Who should not take Micardis HCT?
Do not take Micardis HCT tablets if you:
- have low or no urine output
- are allergic (hypersensitive) to the active ingredients (telmisartan or hydrochlorothiazide) or any of the other ingredients listed at the end of this guide.
What should I tell my healthcare provider before taking Micardis HCT?
Before you take Micardis HCT tablets, tell your doctor if you:
- are pregnant or are planning to become pregnant. See “What is the most important information I should know about Micardis HCT tablets?”
- are breast-feeding or plan to breast-feed. Micardis HCT can pass into your breast milk and may harm your baby. You and your doctor should decide if you will take Micardis HCT or breast-feed. You should not do both. Talk with your doctor about the best way to feed your baby if you take Micardis HCT tablets.
- have been told that you have abnormal body salt (electrolytes) levels in your blood
- have liver problems
- have asthma or history of asthma
- have lupus
- have diabetes
- have kidney problems
- have any other medical conditions
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Also, tell your doctor if you drink alcohol.
Micardis HCT may affect the way other medicines work, and other medicines may affect how Micardis HCT works. Especially tell your doctor if you take:
- aliskiren
- digoxin (Lanoxin)
- lithium (Lithobid, lithium carbonate, lithium citrate)
- other medicines used to treat your high blood pressure or a heart problem
- water pills (diuretic)
- aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs)
- potassium supplements or a salt substitute containing potassium
- medicine used to treat diabetes, including insulin
- narcotic pain medicines
- sleeping pills
- steroid medicine or Adrenocorticotrophic Hormone (ACTH)
- barbiturates
- certain cholesterol lowering medicines (resins that are used for cholesterol reduction, e.g., cholestyramine and colestipol resins)
Ask your doctor if you are not sure if you are taking one of the medicines listed above.
Know the medicines you take. Keep a list of them and show it to your doctor or pharmacist when you get a new medicine.
How should I take Micardis HCT?
- Take Micardis HCT tablets exactly as your doctor tells you to take it.
- Your doctor will tell you how much Micardis HCT to take and when to take it.
- Do not change your dose unless your doctor tells you to.
- Take Micardis HCT once each day.
- Take Micardis HCT tablets with or without food.
- If you take too much Micardis HCT, call your doctor, or go to the nearest hospital emergency room right away.
- Read the instructions for use that come with Micardis HCT. Talk with your doctor if you do not understand the instructions.
What are the possible side effects of Micardis HCT?
Micardis HCT tablets may cause serious side effects, including:
- Injury or death to your unborn baby. See “What is the most important information I should know about Micardis HCT tablets?”
- Low blood pressure (hypotension) is most likely to happen if you also:
- take water pills (diuretics)
- are on a low-salt diet
- get dialysis treatments
- have heart problems
- get sick with vomiting or diarrhea
- do not drink enough fluids
- sweat a lot
If you feel faint or dizzy, lie down and call your doctor right away.
- Kidney problems, which may get worse if you already have kidney disease. You may have changes in your kidney test results, and you may need a lower dose of Micardis HCT tablets. Call your doctor if you get:
- swelling in your feet, ankles, or hands
- unexplained weight gain
Call your doctor right away if you get any of the symptoms listed above.
- Liver problems, which may get worse in people who already have liver problems and take Micardis HCT.
- Eye problems. One of the medicines in Micardis HCT can cause eye problems that may lead to vision loss. Symptoms of eye problems can happen within hours to weeks of starting Micardis HCT. Tell your doctor right away if you have:
- decrease in vision
- eye pain
- Allergic reactions. Tell your doctor right away if you get any of these symptoms:
- swelling of the face, tongue, throat
- difficulty breathing
- Worsening of lupus. Tell your doctor if your lupus gets worse or becomes active while taking Micardis HCT.
- Change in body salts (electrolytes) level in your blood and fluid problems. Your doctor may do tests to check your blood. Call your doctor right away if you have:
- dry mouth
- thirst
- tiredness
- sleepiness
- restlessness
- confusion
- seizures
- fast heartbeats
- weakness
- muscle pain or cramps
- very low urine output
- nausea or vomiting
- Skin Cancer. One of the medicines in Micardis HCT may increase your risk of getting non-melanoma skin cancer. Protect your skin from the sun and undergo regular skin cancer screening when taking Micardis HCT.
The most common side effects of Micardis HCT tablets include:
- upper respiratory tract infections, including sinus pain/congestion and sore throat
- dizziness
- feeling tired
- flu-like symptoms
- back pain
- diarrhea
- nausea
These are not all the possible side effects with Micardis HCT tablets. Tell your doctor if you have any side effect that bothers you or that does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Micardis HCT
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Micardis HCT tablets for a condition for which it was not prescribed. Do not give Micardis HCT tablets to other people, even if they have the same condition you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Micardis HCT tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Micardis HCT tablets that is written for health professionals.
For current prescribing information call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257 or (TTY) 1-800-459-9906.
How should I store Micardis HCT?
- Store Micardis HCT tablets at room temperature 68°F to 77°F (20°C to 25°C).
- Do not remove Micardis HCT tablets from blisters until right before you take them.
Keep Micardis HCT tablets and all medicines out of the reach of children.
What are the ingredients in Micardis HCT?
Active Ingredients: telmisartan and hydrochlorothiazide
Inactive Ingredients: sodium hydroxide, meglumine, povidone, sorbitol, magnesium stearate, lactose monohydrate, microcrystalline cellulose, maize starch, and sodium starch glycolate
The 40 mg/12.5 mg and 80 mg/12.5 mg tablets also contain: ferric oxide red.
The 80 mg/25 mg tablets also contain: ferric oxide yellow.
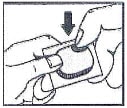
Instructions for use for Micardis HCT
How to open the blister:
- Tear (You may also use scissors to tear the blister apart)
- Peel (Peel off the paper layer from the aluminum foil)
- Push (Push the tablet through the aluminum foil)
Label
PRINCIPAL DISPLAY PANEL – 80 MG/12.5 MG TABLET BLISTER PACK CARTON
- NDC 0597-0044-37
- Micardis® HCT
(telmisartan/
hydrochlorothiazide tablets) - 80 mg/12.5 mg
- Rx only
- 30 tablets – 3 blister cards of 10 tablets each.
Each tablet contains 80 mg of telmisartan and 12.5 mg of
hydrochlorothiazide. - Dosage: Read accompanying prescribing information.
- Keep out of reach of children.
- Important: Moisture sensitive tablets – do not remove
from blisters until immediately before administration. - Boehringer
Ingelheim

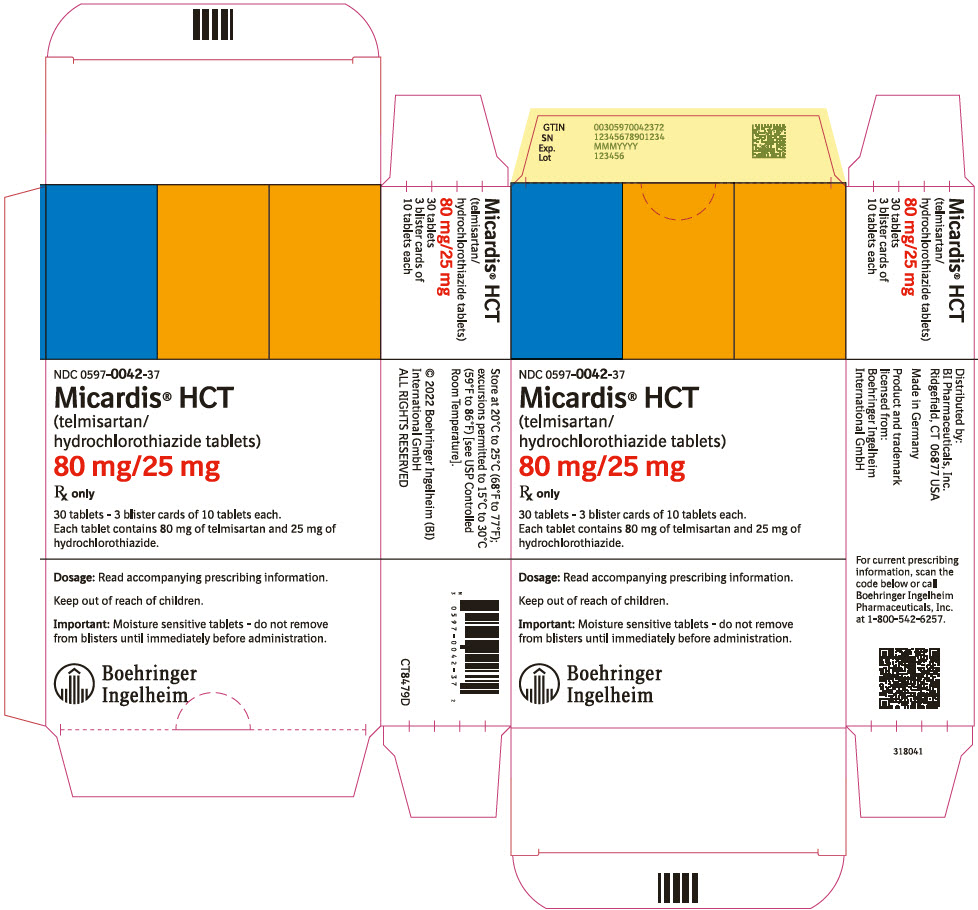
PRINCIPAL DISPLAY PANEL – 80 MG/25 MG TABLET BLISTER PACK CARTON
- NDC 0597-0042-37
- Micardis® HCT
(telmisartan/
hydrochlorothiazide tablets) - 80 mg/25 mg
- Rx only
- 30 tablets – 3 blister cards of 10 tablets each.
Each tablet contains 80 mg of telmisartan and 25 mg of
hydrochlorothiazide. - Dosage: Read accompanying prescribing information.
- Keep out of reach of children.
- Important: Moisture sensitive tablets – do not remove
from blisters until immediately before administration. - Boehringer
Ingelheim
SRC: NLM .
