Lynparza
Generic name: olaparib
Drug class: PARP inhibitors
Medically reviewed by A Ras MD.
What is Lynparza?
Lynparza is a prescription medicine used to treat adults who have advanced ovarian cancer, fallopian tube cancer, or primary peritoneal cancer with a certain type of inherited (germline) or acquired (somatic) abnormal BRCA gene. Lynparza is used alone as maintenance treatment after the cancer has responded to your first treatment with platinum-based chemotherapy. Your healthcare provider will perform a test to make sure that Lynparza is right for you.
It is also used to treat:
- advanced ovarian cancer, fallopian tube cancer or primary peritoneal cancer with a certain type of abnormal BRCA gene or a positive laboratory tumor test for genomic instability called HRD. Lynparza is used in combination with another anti-cancer medicine, bevacizumab, as maintenance treatment after the cancer has responded to your first treatment with platinum-based chemotherapy. Your healthcare provider will perform a test to make sure that Lynparza is right for you.
- ovarian cancer, fallopian tube cancer, or primary peritoneal cancer, as maintenance treatment, when the cancer has come back. Lynparza is used after the cancer has responded to treatment with platinum-based chemotherapy.
- advanced ovarian cancer with a certain type of abnormal inherited BRCA gene, and have received treatment with 3 or more prior types of chemotherapy medicines. Your healthcare provider will perform a test to make sure that Lynparza is right for you.
- human epidermal growth factor receptor 2 (HER2)-negative early breast cancer with a certain type of inherited (germline) abnormal BRCA gene. Lynparza is given after surgery (treatment after surgery is called adjuvant therapy). You should have received chemotherapy medicines before or after surgery to remove the tumor. Your healthcare provider will perform a test to make sure that Lynparza is right for you.
- a certain type of abnormal inherited BRCA gene, human epidermal growth factor receptor 2 (HER2)-negative breast cancer that has spread to other parts of the body (metastatic). You should have received chemotherapy medicines, either before or after your cancer has spread. If you have hormone receptor (HR)-positive disease, you should have been treated with hormonal therapy. Your healthcare provider will perform a test to make sure that Lynparza is right for you.
- metastatic pancreatic cancer with a certain type of abnormal inherited BRCA gene. Lynparza is used as maintenance treatment after your cancer has not progressed on at least 16 weeks of treatment with platinum-based chemotherapy. Your healthcare provider will perform a test to make sure that Lynparza is right for you.
- prostate cancer with certain inherited or acquired abnormal genes called homologous recombination repair (HRR genes). Lynparza is used when the cancer has spread to other parts of the body (metastatic), and no longer responds to a medical or surgical treatment that lowers testosterone, and has progressed after treatment with enzalutamide or abiraterone. Your healthcare provider will perform a test to make sure Lynparza is right for you.
It is not known if Lynparza is safe and effective in children.
Description
Olaparib is a poly (ADP-ribose) polymerase (PARP) inhibitor. The chemical name is 4-[(3-{[4-(cyclopropylcarbonyl)piperazin-1-yl]carbonyl}-4-fluorophenyl)methyl]phthalazin-1(2H)-one. The empirical molecular formula for Lynparza is C24H23FN4O3 and the relative molecular mass is 434.46. It has the following chemical structure:
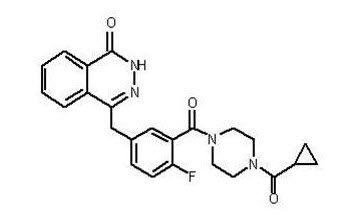
Olaparib is a crystalline solid, is non-chiral and shows pH-independent low solubility across the physiological pH range.
Lynparza (olaparib) tablets for oral use contain 100 mg or 150 mg of olaparib. Inactive ingredients in the tablet core are copovidone, mannitol, colloidal silicon dioxide, and sodium stearyl fumarate. The tablet coating consists of hypromellose, polyethylene glycol 400, titanium dioxide, ferric oxide yellow, and ferrosoferric oxide (150 mg tablet only).
Mechanism of Action
Olaparib is an inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, including PARP1, PARP2, and PARP3. PARP enzymes are involved in normal cellular functions, such as DNA transcription and DNA repair. Olaparib has been shown to inhibit growth of select tumor cell lines in vitro and decrease tumor growth in mouse xenograft models of human cancer, both as monotherapy or following platinum-based chemotherapy. Increased cytotoxicity and anti-tumor activity following treatment with olaparib were noted in cell lines and mouse tumor models with deficiencies in BRCA1/2, ATM, or other genes involved in the homologous recombination repair (HRR) of DNA damage and correlated with platinum response. In vitro studies have shown that olaparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes, resulting in DNA damage and cancer cell death.
What is the most important information I should know about Lynparza?
Lynparza may cause serious side effects, including:
- Bone marrow problems called Myelodysplastic Syndrome (MDS) or Acute Myeloid Leukemia (AML). Some people who have ovarian cancer or breast cancer and who have received previous treatment with chemotherapy, radiotherapy or certain other medicines for their cancer have developed MDS or AML during treatment with Lynparza. MDS or AML may lead to death. If you develop MDS or AML, your healthcare provider will stop treatment with Lynparza.Symptoms of low blood cell counts are common during treatment with Lynparza, but can be a sign of serious bone marrow problems, including MDS or AML. Symptoms may include:
- weakness
- weight loss
- fever
- frequent infections
- blood in urine or stool
- shortness of breath
- feeling very tired
- bruising or bleeding more easilyYour healthcare provider will do blood tests to check your blood cell counts:
- before treatment with Lynparza
- every month during treatment with Lynparza
- weekly if you have low blood cell counts that last a long time. Your healthcare provider may stop treatment with Lynparza until your blood cell counts improve.
- Lung problems (pneumonitis). Tell your healthcare provider if you have any new or worsening symptoms of lung problems, including shortness of breath, fever, cough, or wheezing. Your healthcare provider may do a chest x-ray if you have any of these symptoms. Your healthcare provider may temporarily or completely stop treatment if you develop pneumonitis. Pneumonitis may lead to death.
- Blood clots (Venous Thromboembolic Events). Some people with prostate cancer who take Lynparza along with gonadotropin-releasing hormone (GnRH) analog therapy may develop a blood clot in a deep vein, usually in the leg (venous thrombosis) or a clot in the lung (pulmonary embolism). Tell your healthcare provider if you have any symptoms such as pain or swelling in an extremity, shortness of breath, chest pain, breathing that is more rapid than normal (tachypnea), or heart beats faster than normal (tachycardia). Your healthcare provider will monitor you for these symptoms and may prescribe blood thinner medicine.
What should I tell my healthcare provider before taking Lynparza?
Before taking Lynparza, tell your healthcare provider about all of your medical conditions, including if you:
- have lung or breathing problems
- have kidney problems
- are pregnant, become pregnant, or plan to become pregnant. Lynparza can harm your unborn baby and may cause loss of pregnancy (miscarriage).
- If you are able to become pregnant, your healthcare provider may do a pregnancy test before you start treatment with Lynparza.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with Lynparza and for 6 months after the last dose of Lynparza. Talk to your healthcare provider about birth control methods that may be right for you. Tell your healthcare provider right away if you become pregnant or think you might be pregnant following treatment with Lynparza.
- Males with female partners who are pregnant or able to become pregnant should use effective birth control (contraception) during treatment with Lynparza and for 3 months after the last dose of Lynparza.
- Do not donate sperm during treatment with Lynparza and for 3 months after your final dose.
- are breastfeeding or plan to breastfeed. It is not known if Lynparza passes into your breast milk. Do not breastfeed during treatment with Lynparza and for 1 month after receiving the last dose of Lynparza. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking Lynparza and certain other medicines may affect how Lynparza works and may cause side effects.
How should I take Lynparza?
- Take Lynparza tablets exactly as your healthcare provider tells you.
- Do not change your dose or stop taking Lynparza unless your healthcare provider tells you to. Your healthcare provider may temporarily stop treatment with Lynparza or change your dose of Lynparza if you experience side effects.
- Your healthcare provider will decide how long you stay on treatment.
- Do not take more than 4 Lynparza tablets in 1 day. If you have any questions about Lynparza, please talk to your healthcare provider or pharmacist.
- Take Lynparza by mouth 2 times a day.
- Each dose should be taken about 12 hours apart.
- Swallow Lynparza tablets whole. Do not chew, crush, dissolve, or divide the tablets. Take Lynparza with or without food.
- If you are taking Lynparza for early breast cancer and you have hormone receptor-positive disease, you should continue to take hormonal therapy during your treatment with Lynparza.
- If you are taking Lynparza for prostate cancer and you are receiving gonadotropin-releasing hormone (GnRH) analog therapy, you should continue with this treatment during your treatment with Lynparza unless you have had a surgery to lower the amount of testosterone in your body (surgical castration).
- If you miss a dose of Lynparza, take your next dose at your usual scheduled time. Do not take an extra dose to make up for a missed dose.
- If you take too much Lynparza, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking Lynparza?
Avoid grapefruit, grapefruit juice, Seville oranges and Seville orange juice during treatment with Lynparza since they may increase the level of Lynparza in your blood.
What are the possible side effects of Lynparza?
Lynparza may cause serious side effects.
See “What is the most important information I should know about Lynparza?”
The most common side effects of Lynparza are:
- nausea or vomiting. Tell your healthcare provider if you get nausea or vomiting. Your healthcare provider may prescribe medicines to treat these symptoms.
- tiredness or weakness
- low red blood cell counts
- diarrhea
- loss of appetite
- headache
- low white blood cell counts
- changes in the way food tastes
- cough
- dizziness
- shortness of breath
- indigestion or heartburn
These are not all of the possible side effects of Lynparza.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Lynparza
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Lynparza for a condition for which it was not prescribed. Do not give Lynparza to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Lynparza that is written for health professionals.
How should I store Lynparza?
- Store Lynparza at room temperature, between 68°F to 77°F (20°C to 25°C).
- Store Lynparza in the original bottle to protect it from moisture.
Keep Lynparza and all medicines out of reach of children.
What are the ingredients in Lynparza?
Active ingredient: olaparib
Inactive ingredients: copovidone, mannitol, colloidal silicon dioxide and sodium stearyl fumarate
Tablet coating contains: hypromellose, polyethylene glycol 400, titanium dioxide, ferric oxide yellow and ferrosoferric oxide (150 mg tablet only)
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 100 MG
- NDC 0310-0668-60
- Lynparza®
- (olaparib) tablets
- 100 mg
- Dispense accompanying
- Medication Guide to each patient.
- 60 Tablets
- Rx only
- AstraZeneca
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 150 MG
- NDC 0310-0679-12
- Lynparza®
- (olaparib) tablets
- 150 mg
- Dispense accompanying
- Medication Guide to each patient.
- 120 Tablets
- Rx only
- AstraZeneca

SRC: NLM .
