Lanreotide
Generic name: lanreotide
Brand name: Somatuline Depot
Dosage form: prolonged-release subcutaneous injection
Drug class: Somatostatin and somatostatin analogs
Medically reviewed by A Ras MD.
What is lanreotide?
Lanreotide is a prescription medicine used for the long-term treatment of people with acromegaly when surgery or radiotherapy has not worked well enough or they are not able to have surgery or radiotherapy.
It is also used in for the treatment of adults with a type of cancer known as neuroendocrine tumors, from the gastrointestinal tract or the pancreas (GEP-NETs) that has spread or cannot be removed by surgery.
It is not known if lanreotide is safe and effective in children.
Description
Lanreotide Injection 60 mg/0.2 mL, 90 mg/0.3 mL, and 120 mg/0.5 mL is a prolonged-release formulation for deep subcutaneous injection. It contains the drug substance lanreotide acetate, a synthetic octapeptide with a biological activity similar to naturally occurring somatostatin, water for injection and acetic acid (for pH adjustment).
Lanreotide Injection is available as sterile, ready-to-use, single-dose prefilled syringes containing lanreotide acetate supersaturated bulk solution of 24.6% w/w lanreotide base.
| Each syringe contains: | Lanreotide Injection 60 mg/0.2 mL |
Lanreotide Injection 90 mg/0.3 mL |
Lanreotide Injection 120 mg/0.5 mL |
| Lanreotide acetate | 89.9 mg | 123.2 mg | 156.6 mg |
| Acetic Acid | q.s. | q.s. | q.s. |
| Water for injection | 236.4 mg | 324.1 mg | 411.6 mg |
| Total Weight | 328.9 mg | 450.9 mg | 572.8 mg |
Lanreotide acetate is a synthetic cyclical octapeptide analog of the natural hormone, somatostatin. Lanreotide acetate is chemically known as [cyclo S-S]-3-(2-naphthyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-L-threoninamide, acetate salt. Its molecular weight is 1096.34 (base) and its amino acid sequence is:

The Lanreotide Injection in the prefilled syringe is a white to pale yellow, semi-solid formulation.
Mechanism of Action
Lanreotide, the active component of Lanreotide Injection is an octapeptide analog of natural somatostatin. The mechanism of action of lanreotide is believed to be similar to that of natural somatostatin.
Who should not use lanreotide?
Do not receive lanreotide if you are allergic to lanreotide.
What should I tell my healthcare provider before using lanreotide?
Before you receive lanreotide, tell your healthcare provider about all of your medical conditions, including if you:
- have gallbladder problems.
- have diabetes.
- have heart problems.
- have thyroid problems.
- have kidney problems.
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if lanreotide will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if lanreotide passes into your breast milk. You should not breastfeed if you receive lanreotide and for 6 months after your last dose of lanreotide.
- are a person who can become pregnant. Lanreotide may affect fertility and may affect your ability to become pregnant. Talk to your healthcare provider if this is a concern for you.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Lanreotide and other medicines may affect each other, causing side effects. lanreotide may affect the way other medicines work, and other medicines may affect how lanreotide works. Your dose of lanreotide or your other medicines may need to be changed.
Especially tell your healthcare provider if you take:
- insulin or other diabetes medicines.
- cyclosporine (Gengraf, Neoral, or Sandimmune).
- medicines that lower your heart rate such as beta blockers.
How should I use lanreotide?
- You will receive a lanreotide dose every 4 weeks in your healthcare provider’s office.
- Your healthcare provider may change your dose of lanreotide or the length of time between your injections. Your healthcare provider will tell you how long you need to receive lanreotide.
- Lanreotide is injected deep under the skin of the upper outer area of your buttock. Your injection site should change (alternate) between your right and left buttock from one injection of lanreotide to the next.
- During your treatment with lanreotide for acromegaly, your healthcare provider may do certain blood tests to see if lanreotide is working.
What should I avoid while using lanreotide?
Lanreotide can cause dizziness. If you have dizziness, do not drive a car or operate machinery.
What are the possible side effects of lanreotide?
Lanreotide may cause serious side effects, including:
- Gallstones (cholelithiasis) and complications that can happen if you have gallstones. Gallstones are a serious but common side effect in people who take lanreotide and have acromegaly and GEP-NET. Your healthcare provider may check your gallbladder before and during treatment with lanreotide. Possible complications of gallstones include inflammation and infection of the gallbladder and pancreatitis. Tell your healthcare provider if you get any symptoms of gallstones, including:
- sudden pain in your upper right stomach area (abdomen)
- yellowing of your skin and whites of your eyes
- nausea
- sudden pain in your right shoulder between your shoulder blades
- fever with chills
- Changes in your blood sugar (high blood sugar or low blood sugar). If you have diabetes, test your blood sugar as your healthcare provider tells you to. Your healthcare provider may change your dose of diabetes medicine especially when you first start receiving lanreotide or if your dose of lanreotide changes. High blood sugar is a common side effect in people with GEP-NET.Tell your healthcare provider right away if you have any signs or symptoms of high blood sugar or low blood sugar.
- Signs and symptoms of high blood sugar may include:
- increased thirst
- increased appetite
- nausea
- weakness or tiredness
- urinating more often than normal
- your breath smells like fruit
- Signs and symptoms of low blood sugar may include:
- dizziness or lightheadedness
- sweating
- confusion
- headache
- blurred vision
- slurred speech
- shakiness
- fast heartbeat
- irritability or mood changes
- hunger
- Signs and symptoms of high blood sugar may include:
- Slow heart rate. Tell your healthcare provider right away if you have slowing of your heart rate or if you have symptoms of a slow heart rate, including:
- dizziness or lightheadedness
- fainting or near-fainting
- chest pain
- shortness of breath
- confusion or memory
- weakness, extreme tiredness
- High blood pressure. High blood pressure can happen in people who receive lanreotide and is a common side effect in people with GEP-NET.
- Changes in thyroid function. Lanreotide can cause the thyroid gland to not make enough thyroid hormones that the body needs (hypothyroidism) in people who have acromegaly. Tell your healthcare provider if you have signs and symptoms of low thyroid hormones levels, including:
- fatigue
- weight gain
- a puffy face
- being cold all of the time
- constipation
- dry skin
- thinning, dry hair
- decreased sweating
- depression
The most common side effects of lanreotide in people with acromegaly include:
- diarrhea
- stomach area (abdominal) pain
- nausea
- pain, itching, or a lump at the injection site
The most common side effects of lanreotide in people with GEP-NET include:
- stomach area (abdominal) pain
- muscle and joint aches
- vomiting
- headache
- pain, itching, or a lump at the injection site
Tell your healthcare provider right away if you have signs of an allergic reaction after receiving lanreotide, including:
- swelling of your face, lips, mouth or tongue
- breathing problems
- fainting, dizziness, feeling lightheaded (low blood pressure)
- itching
- flushing or redness of your skin
- rash
- hives
These are not all the possible side effects of lanreotide. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of lanreotide
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not receive lanreotide for a condition for which it was not prescribed. You can ask your healthcare provider for information about lanreotide that is written for health professionals.
What are the ingredients in lanreotide?
Active ingredient: lanreotide acetate
Inactive ingredients: water for injection and acetic acid (for pH adjustment)
For more information, go to WWW.CIPLAUSA.COM or call 1-886-604-3268.
Instructions for use for lanreotide
Lanreotide extended-release gel
The following instructions explain how to inject lanreotide:
1. Read all instructions carefully before starting the injection. Follow this procedure exactly, as it may differ from your past experience.
2. This is a single-use pre-filled syringe with a single-use safety needle with a green needle shield (that cannot be removed) in a clear needle cap.
3. All the medication must be injected slowly over 20 seconds during the use.
4. If you drop or damage the device in any way, please call 1-866-604-3268.
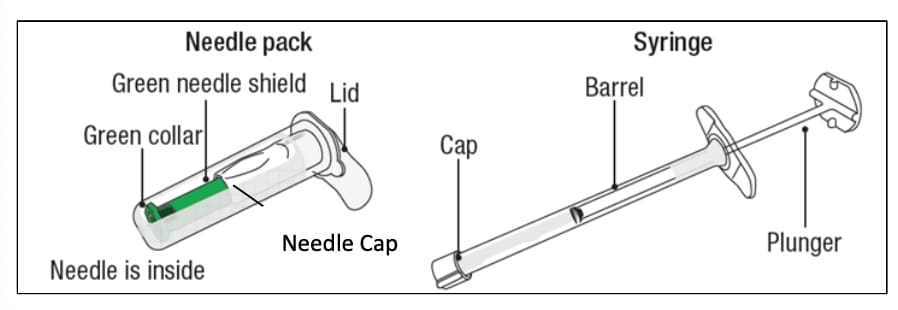
Figure 1
The box includes the following items:
- Sterile needle pack containing Sterile needle
- Sterile Laminated pouch with sterile syringe pre-filled with lanreotide
- Instructions for Use Leaflet
- Prescribing Information Leaflet
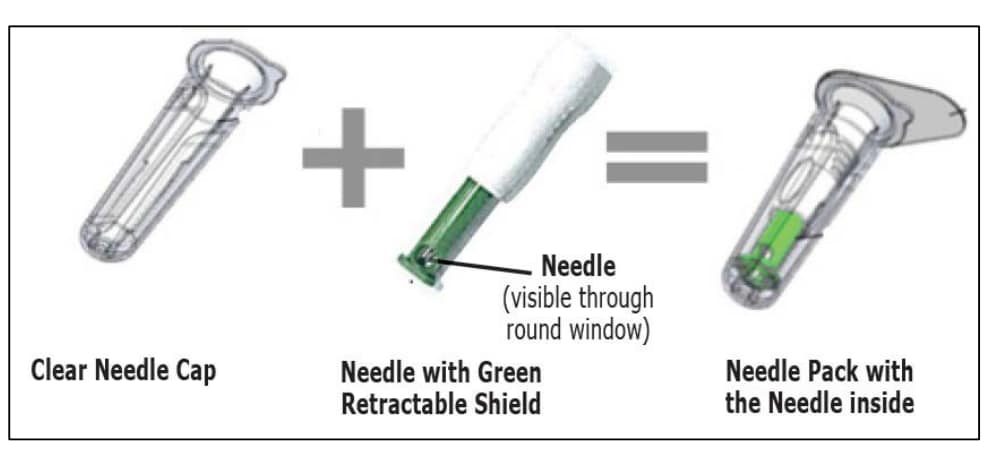
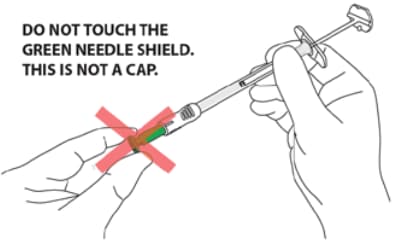
- CAUTION: NEVER TOUCH OR TRY TO OPEN THE GREEN NEEDLE SHIELD throughout the course of operation of the device.
- Green needle shield is NOT a removable cap or cover for the needle.
- Green needle shield will automatically. activate once the injection is complete.
- Green needle shield is a self-operating safety lock mechanism.
- Needle is fully covered by green needle shield.
- Needle is visible only through a small window in the green needle shield.
- Inject medication slowly over 20 seconds.
- DO NOT rush the injection.
- Remove box from refrigerator 30 minutes prior to injecting.
- Product left in its sealed pouch at room temperature (not to exceed 104°F or 40°C) for up to 24 hours may be returned to the refrigerator for continued storage and usage at a later time.
- Stretch out the skin around injection site to make it flat and tight using your thumb and index finger.
- DO NOT pinch the skin around injection site.
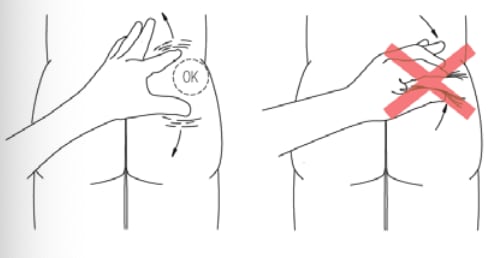
A. Before you start
A1. Remove lanreotide from the refrigerator 30 minutes prior to injecting. Do not open the sterile pouch yet.
Note: Product left in its sealed pouch at room temperature (not to exceed 104° F or 40 °C) for up to 24 hours may be returned to the refrigerator for continued storage and use at a later time.
A2. Wash your hands with soap and dry your hands thoroughly before starting (Figure 3).
| Follow the doctor or institution’s policy on the use of surgical gloves during the procedure |
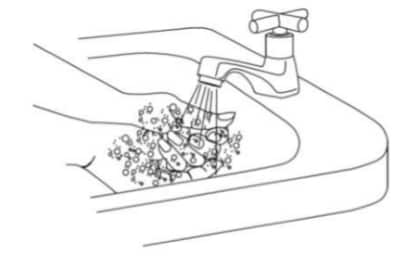
Figure 3
A3. Before opening the sterile pouch, confirm that it. is intact, and that the medication has not expired. The Syringe is sterile only if the pouch is sealed and undamaged. Do not use if the pouch is opened, tampered with or damaged. The expiry date is printed on the outer carton and the pouch – Do not. use if the medication has expired. (Figure 4).
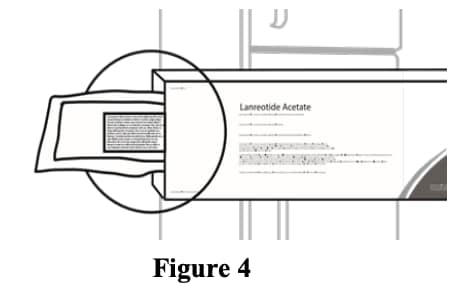
Figure 4
A4. Make sure there is a clean surface for preparation.
Find a clean, comfortable area for the patient to relax during procedure (Figure 5).
It’s important that the patient remains as still as possible during the injection.
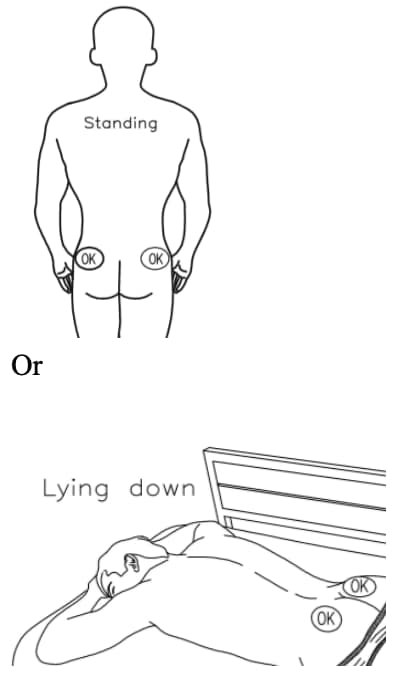
Figure 5
A5. Choose injection site – the sites are upper. outer areas of the buttock as shown below. It is very important that you only inject in one of the areas marked OK in the picture (Figure 6).
| Alternate the injection site between the right. and left side each time an injection of lanreotide is administered. |
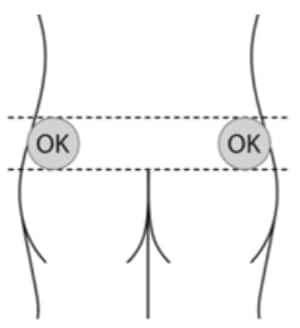
Figure 6
A6. Prepare the injection site by cleaning it with. alcohol wipes (Figure 7).
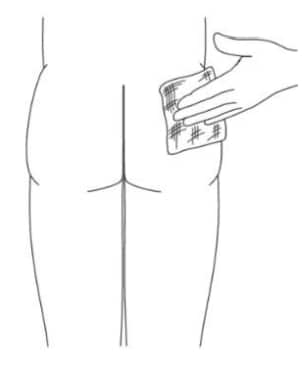
Figure 7
A7. Tear open the sterile pouch and take out the sterile pre-filled syringe (Figure 8).
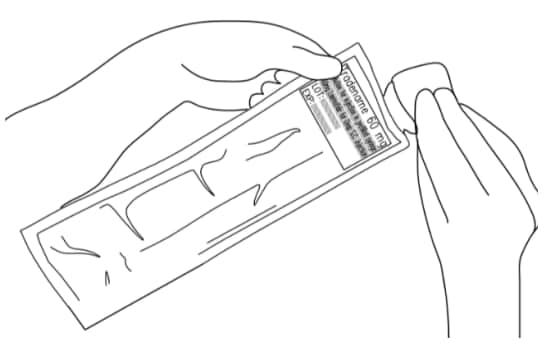
Figure 8
B. Prepare the syringe
B1: Open the sterile needle cap (Figure 9)
- The needle is sterile only if the needle cap is sealed and undamaged. Do not use if the needle cap is opened, tampered with or damaged.
- Hold the clear needle cap and pull the lid off.
- DO NOT TOUCH THE OPEN END OF THE NEEDLE CAP TO MAINTAIN STERILITY.
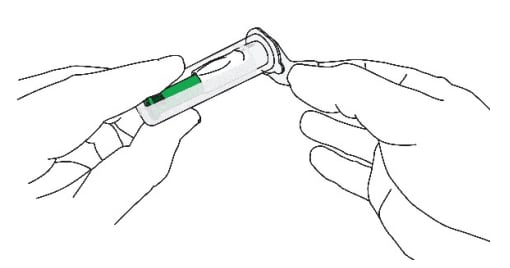
Figure 9
B2: Remove the cap from the sterile syringe (Figure 10)
- With one hand, hold the syringe barrel steady (not the plunger).
- With the other hand, remove the cap by twisting it.
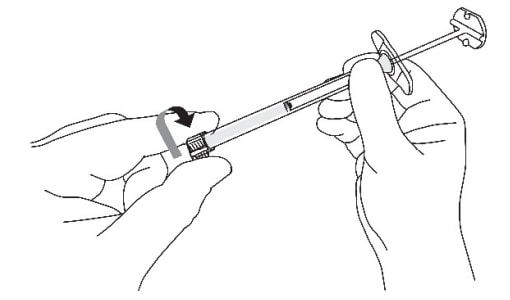
Figure 10
B3: Prepare the assembly (Figure 11)
- Hold the needle cap with one hand and the syringe barrel (not the plunger) with the other
- Carefully insert the open end of the syringe into the open end of the needle cap
- Twist the syringe barrel clockwise until it is tight to make sure that the syringe is well connected to the safety needle.
- ENSURE THAT BOTH PARTS OF THE DEVICE (SYRINGE AND NEEDLE) ARE COMPLETELY CONNECTED.
The assembly is fully locked when you cannot turn it any further.
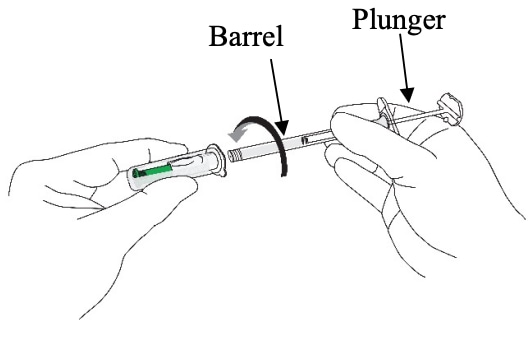
Figure 11
B4: Remove the needle from the needle
- Hold the syringe barrel (not the plunger).
- Pull the needle straight out from the needle. cap without twisting or turning (Figure 12).
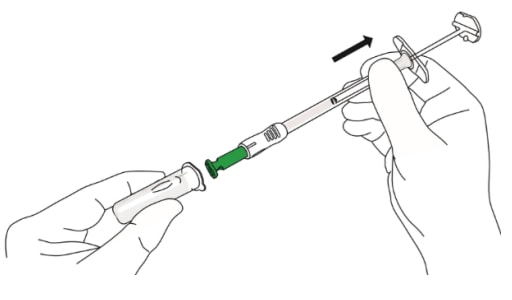
Figure 12
CAUTION: NEVER TOUCH THE GREEN NEEDLE SHIELD. THERE IS A RISK OF NEEDLE STICK INJURY. (Figure 13)
- Green needle shield is a self-operating safety lock mechanism and is not a removable cover or cap for the needle. It will activate once the injection is complete.
- The needle is fully covered by the green shield and is visible only through the small round window at the end of the shield.
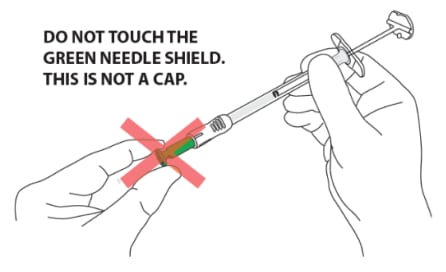
Figure 13
C. Perform injection
C1: Position the syringe assembly
- Stretch out the skin around the injection site. to make it flat and tight using your thumb and index finger. (Figure 14)
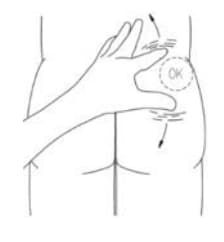
Figure 14
DO NOT pinch the skin (Figure 15)
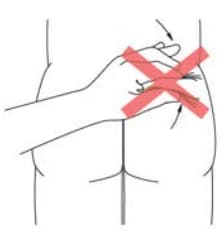
Figure 15
- Hold the assembly by the lower part of the syringe barrel (not the plunger) with your other hand.
- Position the syringe assembly at a 90-degree angle to the skin. The green collar at the bottom of the green shield should be perpendicular to the injection site (Figures 16 and 17)
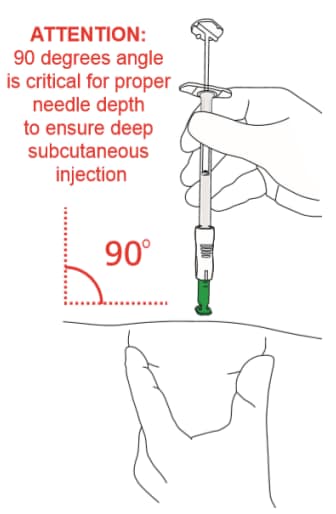
Figure 16
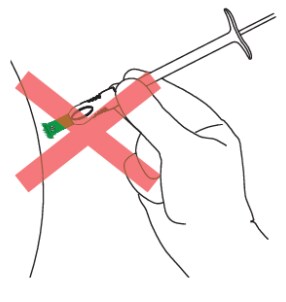
Figure 17
C2: Insert the needle (Figure 18)
- Holding the skin flat and tight, push the syringe assembly firmly into the skin.
- Do NOT pinch the skin.
- Keep pushing until you reach a “hard stop” and only the green collar at the end of the green needle shield remains visible.
- Do NOT push the plunger yet.
- DO NOT aspirate.
- Keep pressing against the skin.
- During this step you should not see the needle.
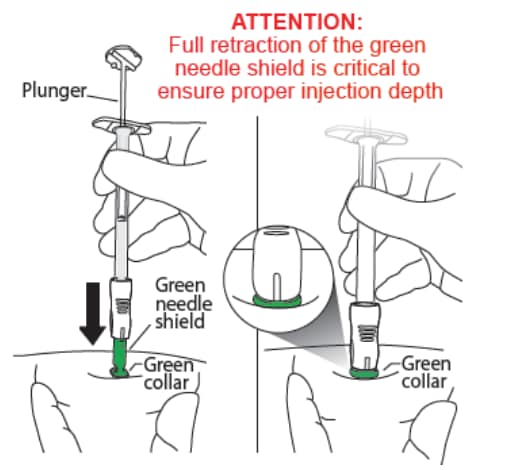
Figure 18
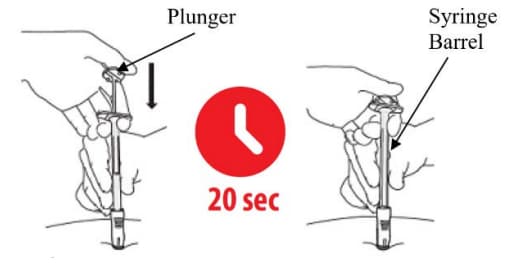
C3: Push the top of the plunger (Figure 19)
- Keep holding your hand on the syringe barrel to maintain a 90- degree angle throughout the injection.
- While keeping the syringe pressed against the skin, slowly press down the plunger. You may want to use both hands while applying pressure during the injection of drug.
- Continue slowly pushing the plunger over 20 seconds until the Plunger top touches the syringe end.
- DO NOT rush the injection. The medication is thicker and harder to push than you might expect. Pushing the plunger too fast may cause discomfort to the patient.
- While depressing the plunger, slowly count to 20 and CONTINUE STEADY PRESSURE on the plunger. You may find it helpful to say:
- a. “1 one-thousand”
- b. “2 one-thousand”
- c. “3 one-thousand” up to “20 one-thousand”
- During this step, as the needle is lowering, the green needle shield will retract. You should only see the green collar of the green needle shield.
- During this step you should not see the needle.
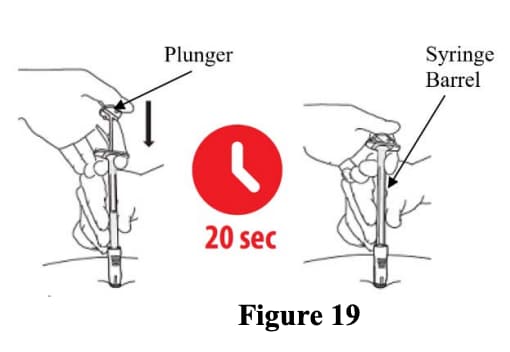
Figure 19
D. Remove and throw away the syringe assembly
D1: Remove from the skin (Figure 20)
- Lift the syringe straight up and away from the body.
- The green needle shield will return to its original position and will fully cover the needle.
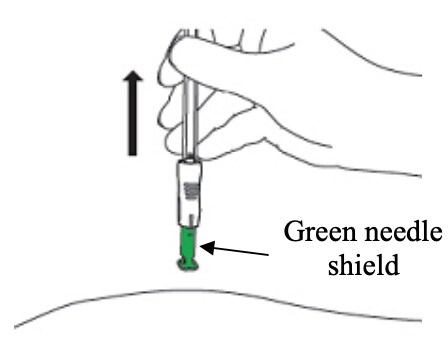
Figure 20
D2: Apply gentle pressure (Figure 21)
- Apply gentle pressure to the injection site. with a dry cotton ball or sterile gauze to prevent any bleeding.
- Do NOT rub or massage the injection site after administration.
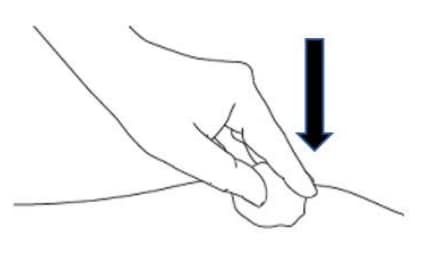
Figure 21
D3: Check needle (Figure 22)
- Check through the green needle shield’s round windows that the needle was not damaged during administration.
- If any damage or malfunction to the needle is suspected please contact 1-866-604-3268
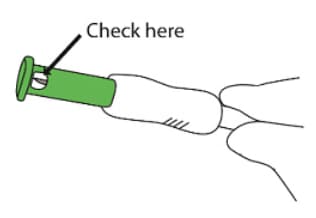
Figure 22
D4: Throw away / Disposal (Figure 23)
- DO NOT remove needle from syringe
- Dispose of complete product in sharps disposal container
- Do not dispose of the syringe or needle in the regular trash.
- The syringe and needle are not reusable.
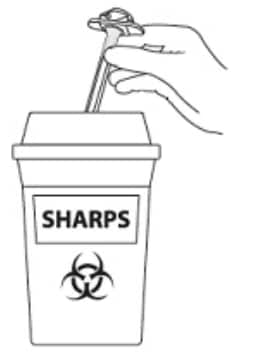
Label
PRINCIPAL DISPLAY PANEL – 60 MG/0.2 ML CARTON LABEL
- NDC 69097-880-67
- Rx Only
- Lanreotide Injection
- 60 mg*/0.2 mL
- For deep subcutaneous injection
- Single dose only. Discard unused portion.
- Lanreotide Injection should be administered by a healthcare professional.
- Leave at room temperature for 30 minutes before administration.
- CONTENTS: This box contains one (1) pre-filled syringe and one (1) safety needle.
Cipla

PRINCIPAL DISPLAY PANEL – 90 MG/0.3 ML CARTON LABEL
- NDC 69097-890-67
- Rx Only
- Lanreotide Injection
- 90 mg*/0.3 mL
- For deep subcutaneous injection
- Single dose only. Discard unused portion.
- Lanreotide Injection should be administered by a healthcare professional.
- Leave at room temperature for 30 minutes before administration.
- CONTENTS: This box contains one (1) pre-filled syringe and one (1) safety needle.
- Cipla

PRINCIPAL DISPLAY PANEL – 120 MG/0.5 ML CARTON LABEL
- NDC 69097-870-67
- Rx Only
- Lanreotide Injection
- 120 mg*/0.5 mL
- For deep subcutaneous injection
- Single dose only. Discard unused portion.
- Lanreotide Injection should be administered by a healthcare professional.
- Leave at room temperature for 30 minutes before administration.
- CONTENTS: This box contains one (1) pre-filled syringe and one (1) safety needle.
- Cipla

SRC: NLM .
