Kadian
Generic name: morphine (oral)
Brand names: Kadian, MS Contin
Drug class: Narcotic analgesics
Medically reviewed by A Ras MD.
What is Kadian?
Kadian is strong prescription pain medicine that contains an opioid (narcotic) that is used to manage pain severe enough to require daily around-the-clock, long-term treatment with an opioid, when other pain treatments such as non-opioid pain medicines or immediate-release opioid medicines do not treat your pain well enough or you cannot tolerate them.
It is a long-acting (extended-release) opioid pain medicine that can put you at risk for overdose and death. Even if you take your dose correctly as prescribed you are at risk for opioid addiction, abuse, and misuse that can lead to death.
Not for use to treat pain that is not around-the-clock.
Description
KADIAN (morphine sulfate) extended-release capsules, an opioid agonist, are for oral use and contain pellets of morphine sulfate.
Each KADIAN extended-release capsule contains either 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, 80 mg, 100 mg, or 200 mg of morphine sulfate, USP and the following inactive ingredients common to all strengths: hypromellose, ethylcellulose, methacrylic acid copolymer, polyethylene glycol, diethyl phthalate, talc, corn starch, and sucrose.
The capsule shells contain gelatin, silicon dioxide, sodium lauryl sulfate, titanium dioxide, and black ink, D&C red #28, FD&C blue #1 (10 mg), D&C yellow #10 (20 mg), FD&C red #3, FD&C blue #1 (30 mg), D&C yellow #10, FD&C blue #1, FD&C red #3 (40 mg), D&C red #28, FD&C red #40, FD&C blue #1 (50 mg), D&C red #28, FD&C red #40, FD&C blue #1 (60 mg), FD&C blue #1, FD&C red #40, FD&C yellow #6 (80 mg), D&C yellow #10, FD&C blue #1 (100 mg) black iron oxide, yellow iron oxide, red iron oxide (200 mg). The imprint ink contains black iron oxide, potassium hydroxide, propylene glycol, and shellac.
The chemical name of morphine sulfate is 7,8-didehydro-4,5 α- epoxy-17-methyl-morphinan-3,6 α-diol sulfate (2:1) (salt) pentahydrate. The empirical formula is (C17H19NO3)2•H2SO4•5H2O and its molecular weight is 758.85.
Morphine sulfate is an odorless, white, crystalline powder with a bitter taste. It has a solubility of 1 in 21 parts of water and 1 in 1000 parts of alcohol, but is practically insoluble in chloroform or ether. The octanol: water partition coefficient of morphine is 1.42 at physiologic pH and the pKb is 7.9 for the tertiary nitrogen (mostly ionized at pH 7.4). Its structural formula is:

Mechanism of Action
Morphine is a full opioid agonist and is relatively selective for the mu-opioid receptor, although it can bind to other opioid receptors at higher doses. The principal therapeutic action of morphine is analgesia. Like all full opioid agonists, there is no ceiling effect for analgesia with morphine. Clinically, dosage is titrated to provide adequate analgesia and may be limited by adverse reactions, including respiratory and CNS depression.
The precise mechanism of the analgesic action is unknown. However, specific CNS opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and are thought to play a role in the analgesic effects of this drug.
What is the most important information I should know about Kadian?
- Get emergency help right away if you take too much Kadian (overdose). When you first start taking Kadian, when your dose is changed, or if you take too much (overdose), serious or life threatening breathing problems that can lead to death may occur.
- Taking Kadian with other opioid medicines, benzodiazepines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, decreased awareness, breathing problems, coma, and death.
- Never give anyone else your Kadian. They could die from taking it. Selling or giving away Kadian is against the law.
- Store Kadian securely, out of sight and reach of children, and in a location not accessible by others, including visitors to the home.
Who should not take Kadian?
Do not take Kadian if you have:
- severe asthma, trouble breathing, or other lung problems.
- a bowel blockage or have narrowing of the stomach or intestines.
What should I tell my healthcare provider before taking Kadian?
Before taking Kadian, tell your healthcare provider if you have a history of:
- head injury
- seizures
- liver, kidney, thyroid problems
- problems urinating
- pancreas or gallbladder problems
- abuse of street or prescription drugs, alcohol addiction, or mental health problems.
Tell your healthcare provider if you are:
- pregnant or planning to become pregnant. Prolonged use of Kadian during pregnancy can cause withdrawal symptoms in your newborn baby that could be life-threatening if not recognized and treated.
- breastfeeding. Not recommended during treatment with Kadian. It may harm your baby.
- taking prescription or over-the-counter medicines, vitamins, or herbal supplements. Taking Kadian with certain other medicines can cause serious side effects.
How should I take Kadian?
When taking Kadian:
- Do not change your dose. Take Kadian exactly as prescribed by your healthcare provider. Use the lowest dose possible for the shortest time needed.
- Take your prescribed dose every 12 or 24 hours at the same time every day. Do not take more than your prescribed dose in 24 hours. If you miss a dose, take your next dose at your usual time.
- Swallow Kadian whole. Do not cut, break, chew, crush, dissolve, snort, or inject Kadian because this may cause you to overdose and die.
- You should not receive Kadian through a nasogastric tube.
- If you cannot swallow Kadian capsules, see the detailed Instructions for Use.
- Call your healthcare provider if the dose you are taking does not control your pain.
- Do not stop taking Kadian without talking to your healthcare provider.
- Dispose of expired, unwanted, or unused Kadian by promptly flushing down the toilet, if a drug take-back option is not readily available. Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
What should I avoid while taking Kadian?
While taking Kadian Do Not:
- Drive or operate heavy machinery, until you know how Kadian affects you. Kadian can make you sleepy, dizzy, or lightheaded.
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol. Using products containing alcohol during treatment with Kadian may cause you to overdose and die.
What are the possible side effects of Kadian?
- constipation, nausea, sleepiness, vomiting, tiredness, headache, dizziness, abdominal pain. Call your healthcare provider if you have any of these symptoms and they are severe.
Get emergency medical help if you have:
- trouble breathing, shortness of breath, fast heartbeat, chest pain, swelling of your face, tongue, or throat, extreme drowsiness, light-headedness when changing positions, feeling faint, agitation, high body temperature, trouble walking, stiff muscles, mental changes such as confusion.
These are not all the possible side effects of Kadian. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What are the ingredients in Kadian?
Active ingredients: morphine sulfate
Inactive ingredients:
All capsules contain: hypromelloses, ethylcelluloses, methacrylic acid – methyl methacrylate copolymer (1:1), unspecified polyethylene glycol, diethyl phthalate, talc, corn starch, sucrose, gelatin, silicon dioxide, sodium lauryl sulfate, titanium dioxide, shellac, propylene glycol, potassium hydroxide, ferrosoferric oxide.
In addition the following capsules include:
Light blue 10mg capsule: d&c red no. 28 and fd&c blue no. 1.
Yellow 20mg capsule: d&c yellow no. 10.
Purple (violet) 30mg capsule: fd&c red no. 3. and fd&c blue no.1
Yellow, blue (violet) 40mg capsule: fd&c red no. 3, fd&c blue no.1 and d&c yellow no. 10
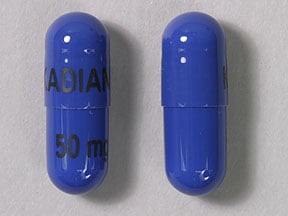
Blue 50mg capsule: fd&c red no. 40, fd&c blue no. 1 and d&c red no. 28
Pink 60mg capsule: fd&c red no. 40, fd&c blue no. 1 and d&c red no. 28
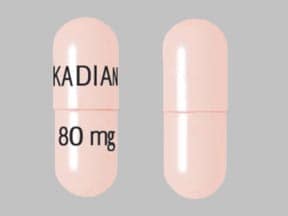
Light orange 80mg capsule: fd&c red no. 40, fd&c yellow no. 6 and fd&c blue no. 1
Green 100mg capsule: d&c yellow no. 10 and fd&c blue no. 1
Light brown 200mg capsule: ferric oxide red and ferric oxide yellow
Label
PRINCIPAL DISPLAY PANEL
- NDC 0023-6012-60
KADIAN®
Morphine Sulfate
Extended-Release Capsules
20 mg
60 Capsules
Rx only

PRINCIPAL DISPLAY PANEL
- NDC 0023-6013-60
KADIAN®
Morphine Sulfate
Extended-Release Capsules
30 mg
60 Capsules
Rx only

PRINCIPAL DISPLAY PANEL
- NDC 0023-6015-60
KADIAN®
Morphine Sulfate
Extended-Release Capsules
50 mg
60 Capsules
Rx only

PRINCIPAL DISPLAY PANEL
- NDC 0023-6017-60
KADIAN®
Morphine Sulfate
Extended-Release Capsules
80 mg
60 Capsules
Rx only

PRINCIPAL DISPLAY PANEL
- NDC 0023-6018-60
KADIAN®
Morphine Sulfate
Extended-Release Capsules
100 mg
60 Capsules
Rx only

PRINCIPAL DISPLAY PANEL
- NDC 0023-6019-60
KADIAN®
Morphine Sulfate
Extended-Release Capsules
200 mg
60 Capsules
Rx only

SRC: NLM .