Intuniv
Generic name: guanfacine
Drug class: Antiadrenergic agents, centrally acting
Medically reviewed by A Ras MD.
What is Intuniv?
Intuniv is a prescription medicine used to treat the symptoms of attention deficit hyperactivity disorder (ADHD). Intuniv may be used alone or with ADHD stimulant medicines.
Intuniv is not a central nervous system (CNS) stimulant.
It is not known if Intuniv is safe and effective in children younger than 6 years of age.
Description
INTUNIV® is a once-daily, extended-release formulation of guanfacine hydrochloride (HCl) in a matrix tablet formulation for oral administration only. The chemical designation is N-amidino-2-(2,6-dichlorophenyl) acetamide monohydrochloride. The molecular formula is C9H9Cl2 N3O∙HCl corresponding to a molecular weight of 282.55. The chemical structure is:
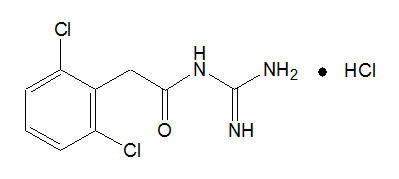
Guanfacine HCl is a white to off-white crystalline powder, sparingly soluble in water (approximately 1 mg/mL) and alcohol and slightly soluble in acetone. The only organic solvent in which it has relatively high solubility is methanol (>30 mg/mL). Each tablet contains guanfacine HCl equivalent to 1 mg, 2 mg, 3 mg, or 4 mg of guanfacine base. The tablets also contain hypromellose, methacrylic acid copolymer, lactose, povidone, crospovidone, microcrystalline cellulose, fumaric acid, and glyceryl behenate. In addition, the 3-mg and 4-mg tablets also contain green pigment blend PB-1763.
Mechanism of Action
Guanfacine is a central alpha2A-adrenergic receptor agonist. Guanfacine is not a central nervous system (CNS) stimulant. The mechanism of action of guanfacine in ADHD is not known.
Who should not take Intuniv?
Do not take Intuniv if you are allergic to guanfacine or any of the ingredients in Intuniv. See the end of this leaflet for a complete list of ingredients in Intuniv.
What should I tell my healthcare provider before taking Intuniv?
Before you take Intuniv, tell your doctor if you:
- have heart problems or a low heart rate
- have fainted
- have low or high blood pressure
- have liver or kidney problems
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if Intuniv will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if Intuniv passes into your breast milk. Talk to your doctor about the best way to feed your baby while taking Intuniv.
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Intuniv may affect the way other medicines work, and other medicines may affect how Intuniv works.
Especially tell your doctor if you take:
- ketoconazole
- medicines that can affect enzyme metabolism
- high blood pressure medicine
- sedatives
- benzodiazepines
- barbiturates
- antipsychotics
Ask your doctor or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I take Intuniv?
- Take Intuniv exactly as your doctor tells you.
- Your doctor may change your dose. Do not change your dose of Intuniv without talking to your doctor.
- Do not stop taking Intuniv without talking to your doctor.
- Try not to miss your dose of Intuniv. If you miss a dose of Intuniv, take the next dose at your regular time. If you miss 2 or more doses, talk to your doctor, as you may need to restart Intuniv with a lower dose.
- Do not take a double dose to make up for a missed dose.
- Intuniv should be taken 1 time a day in the morning or in the evening, either alone or in combination with an ADHD stimulant medicine that your doctor may prescribe. Your doctor will tell you when to take Intuniv and when to take your ADHD stimulant medication.
- Intuniv should be swallowed whole with a small amount of water, milk, or other liquid.
- Do not crush, chew, or break Intuniv. Tell your doctor if you cannot swallow Intuniv whole.
- Do not take Intuniv with a high-fat meal.
- Your doctor will check your blood pressure and heart rate while you take Intuniv.
- If you take too much Intuniv, call your local Poison Control Center at 1-800-222-1222 or go to the nearest emergency room right away.
What should I avoid while taking Intuniv?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how Intuniv affects you. Intuniv can slow your thinking and motor skills.
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking Intuniv until you talk with your doctor. Intuniv taken with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not become dehydrated or overheated. This may increase your chance of having low blood pressure or fainting while taking Intuniv.
- Do not suddenly stop Intuniv. Tell your healthcare provider if you have been vomiting and cannot take Intuniv, you may be at risk for rebound hypertension.
What are the possible side effects of Intuniv?
Intuniv may cause serious side effects including:
- low blood pressure
- low heart rate
- fainting
- sleepiness
- increased blood pressure and heart rate after suddenly stopping Intuniv (rebound hypertension). Suddenly stopping Intuniv can cause increased blood pressure and heart rate and other withdrawal symptoms such as:
- headache
- confusion
- nervousness
- agitation
- tremors
If these symptoms continue to get worse and are left untreated, it could lead to a very serious condition including very high blood pressure, feeling very sleepy or tired, severe headache, vomiting, vision problems, seizures.
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Intuniv include:
- sleepiness
- tiredness
- trouble sleeping
- low blood pressure
- nausea
- stomach pain
- dizziness
- dry mouth
- irritability
- vomiting
- slow heart rate
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Intuniv. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General Information about the safe and effective use Intuniv
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use Intuniv for a condition for which it was not prescribed. Do not give Intuniv to other people, even if they have the same symptoms that you have. It may harm them.
This guide summarizes the most important information about Intuniv. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Intuniv that is written for health professionals.
For more information, go to www.Intuniv.com or call 1-800-828-2088.
How should I store Intuniv?
- Store Intuniv between 68°F to 77°F (20°C to 25°C)
Keep Intuniv and all medicines out of the reach of children.
What are the ingredients in Intuniv?
Active ingredient: guanfacine hydrochloride
Inactive ingredients: hypromellose, methacrylic acid copolymer, lactose, povidone, crospovidone, microcrystalline cellulose, fumaric acid, and glycerol behenate. In addition, the 3 mg and 4 mg tablets also contain green pigment blend PB-1763.
Label
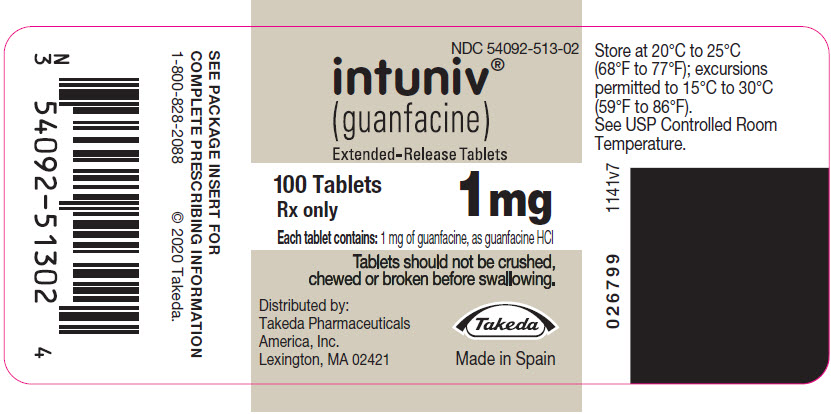
PRINCIPAL DISPLAY PANEL – 1 MG TABLET BOTTLE LABEL
- NDC 54092-513-02
- intuniv®
(guanfacine)
Extended-Release Tablets - 100 Tablets
1 mg
Rx only - Each tablet contains: 1 mg of guanfacine, as guanfacine HCl
- Tablets should not be crushed,
chewed or broken before swallowing. - Distributed by:
Takeda Pharmaceuticals
America, Inc.
Lexington, MA 02421 - Takeda
- Made in Spain
![]()
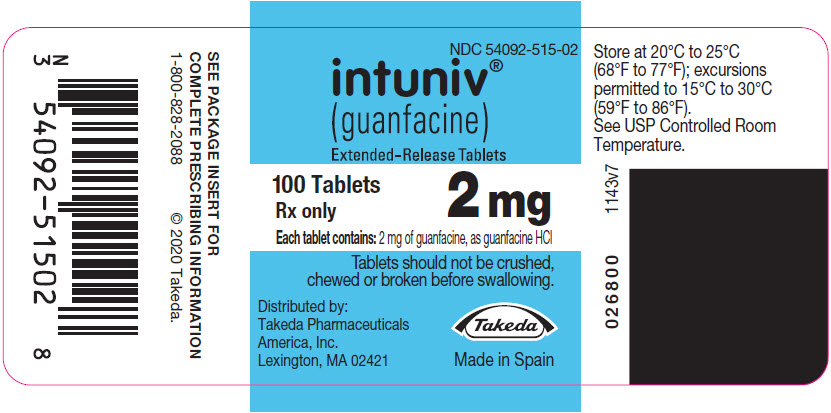
PRINCIPAL DISPLAY PANEL – 2 MG TABLET BOTTLE LABEL
- NDC 54092-515-02
- intuniv®
(guanfacine)
Extended-Release Tablets - 100 Tablets
2 mg
Rx only - Each tablet contains: 2 mg of guanfacine, as guanfacine HCl
- Tablets should not be crushed,
chewed or broken before swallowing. - Distributed by:
Takeda Pharmaceuticals
America, Inc.
Lexington, MA 02421 - Takeda
- Made in Spain
![]()
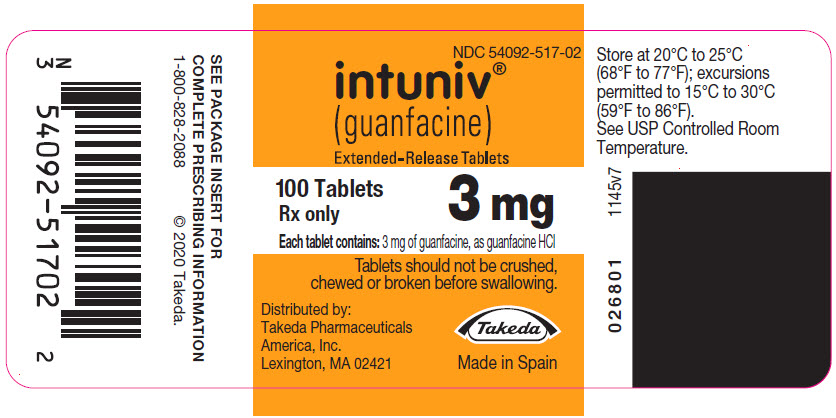
PRINCIPAL DISPLAY PANEL – 3 MG TABLET BOTTLE LABEL
- NDC 54092-517-02
- intuniv®
(guanfacine)
Extended-Release Tablets - 100 Tablets
3 mg
Rx only - Each tablet contains: 3 mg of guanfacine, as guanfacine HCl
- Tablets should not be crushed,
chewed or broken before swallowing. - Distributed by:
Takeda Pharmaceuticals
America, Inc.
Lexington, MA 02421 - Takeda
- Made in Spain
![]()
PRINCIPAL DISPLAY PANEL – 4 MG TABLET BOTTLE LABEL
- NDC 54092-519-02
- intuniv®
(guanfacine)
Extended-Release Tablets - 100 Tablets
4 mg
Rx only - Each tablet contains: 4 mg of guanfacine, as guanfacine HCl
- Tablets should not be crushed,
chewed or broken before swallowing. - Distributed by:
Takeda Pharmaceuticals
America, Inc.
Lexington, MA 02421 - Takeda
- Made in Spain

SRC: NLM .
