Ilaris
Generic name: canakinumab
Drug class: Interleukin inhibitors
Medically reviewed by A Ras MD.
What is Ilaris?
Ilaris is a prescription medicine injected by your healthcare provider just below the skin (subcutaneous) used to treat the following Periodic Fever Syndromes, adults and children 4 years of age and older who have auto-inflammatory diseases called Cryopyrin-Associated Periodic Syndromes (CAPS), including Familial Cold Auto-inflammatory Syndrome (FCAS), Muckle-Wells Syndrome (MWS)
It is also used to treat adults and children who have an auto-inflammatory disease called Tumor Necrosis Factor Receptor Associated Periodic Syndrome (TRAPS), Adults and children who have an auto-inflammatory disease called Hyperimmunoglobulin D Syndrome (HIDS) (also known as Mevalonate Kinase Deficiency (MKD), Adults and children who have an auto-inflammatory disease called Familial Mediterranean Fever (FMF), Still’s disease, including Adult-Onset Still’s Disease (AOSD) and Systemic Juvenile Idiopathic Arthritis (SJIA) in children 2 years of age and older.
It is not known if Ilaris is safe and effective when used to treat SJIA in children under 2 years of age or when used to treat CAPS in children under 4 years of age.
What are Periodic Fever Syndromes?
Periodic Fever Syndromes is the name for several different autoinflammatory diseases, including CAPS, TRAPS, HIDS/MKD, and FMF. People with these diseases cannot keep certain chemicals made by their body (interleukin-1 beta, also called IL-1β) at the correct level. All these diseases have symptoms that often come and go, with irritated body parts (inflammation) and elevated body temperature (fever). These conditions have a dysregulation of IL-1β production and share similar clinical features of recurrent episodes of inflammation and fever; such as rash, headache, pain (mostly in the joints, belly, eyes, muscles), fatigue, inflammation of other organs, such as heart, lungs, spleen, and brain.
What is Still’s Disease (AOSD and SJIA)?
Still’s disease (which is referred to as AOSD in adults and SJIA in children) is an autoinflammatory disorder which can be caused by having too much or being too sensitive to certain proteins, including interleukin-1β (IL-1β), and can lead to symptoms such as fever, rash, headache, feeling very tired (fatigue), or painful joints and muscles.
What is Macrophage Activation Syndrome (MAS)?
MAS is a syndrome associated with Still’s disease and some other autoinflammatory diseases like HIDS/MKD that can lead to death. Tell your healthcare provider right away if your AOSD or SJIA symptoms get worse or if you have any of these symptoms of an infection:
- a fever lasting longer than 3 days.
- a cough that does not go away.
- redness in one part of your body.
- warm feeling or swelling of your skin.
Description
Canakinumab is a recombinant, human anti-human-IL-1β monoclonal antibody that belongs to the IgG1/κ isotype subclass. It is expressed in a murine Sp2/0-Ag14 cell line and comprised of two 447- (or 448-) residue heavy chains and two 214-residue light chains, with a molecular mass of 145157 Daltons when deglycosylated. Both heavy chains of canakinumab contain oligosaccharide chains linked to the protein backbone at asparagine 298 (Asn 298).
The biological activity of canakinumab is measured by comparing its inhibition of IL-1β-dependent expression of the reporter gene luciferase to that of a canakinumab internal reference standard, using a stably transfected cell line.
ILARIS Injection
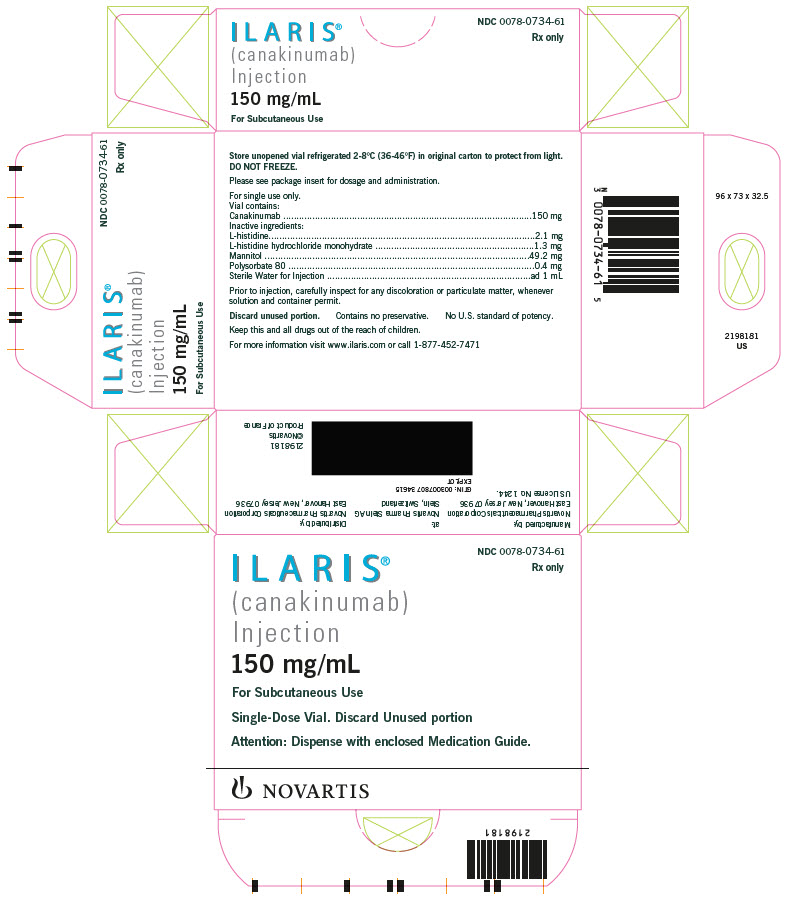
ILARIS (canakinumab) Injection is supplied as a sterile, preservative-free, clear to opalescent, colorless to slightly brownish-yellow solution for subcutaneous injection in a single-dose, glass vial with coated stopper and aluminum flip-off cap. Each vial delivers 1 mL containing 150 mg canakinumab, L-histidine (2.1 mg), L-histidine HCl monohydrate (1.3 mg), mannitol (49.2 mg), polysorbate 80 (0.4 mg), and Sterile Water for Injection.
Mechanism of Action
Canakinumab is a human monoclonal anti-human IL-1β antibody of the IgG1/κ isotype. Canakinumab binds to human IL-1β and neutralizes its activity by blocking its interaction with IL-1 receptors, but it does not bind IL-1α or IL-1 receptor antagonist (IL-1ra).
CAPS refer to rare genetic syndromes generally caused by mutations in the NLRP-3 [nucleotide-binding domain, leucine rich family (NLR), pyrin domain containing 3] gene (also known as Cold-Induced Autoinflammatory Syndrome-1 [CIAS1]). CAPS disorders are inherited in an autosomal dominant pattern with male and female offspring equally affected. Features common to all disorders include fever, urticaria-like rash, arthralgia, myalgia, fatigue, and conjunctivitis.
The NLRP-3 gene encodes the protein cryopyrin, an important component of the inflammasome. Cryopyrin regulates the protease caspase-1 and controls the activation of IL-1β. Mutations in NLRP-3 result in an overactive inflammasome resulting in excessive release of activated IL-1β that drives inflammation. Still’s disease is a severe autoinflammatory disease, driven by innate immunity by means of proinflammatory cytokines such as IL-1β.
What is the most important information I should know about Ilaris?
Ilaris can cause serious side effects, including:
- Increased risk of serious infections. Ilaris can lower the ability of your immune system to fight infections. Your healthcare provider should:
- test you for tuberculosis (TB) before you receive Ilaris
- monitor you closely for symptoms of TB during treatment with Ilaris
- check you for symptoms of any type of infection before, during, and after your treatment with Ilaris
Tell your healthcare provider right away if you have any symptoms of an infection such as fever, sweats or chills, cough, flu-like symptoms, weight loss, shortness of breath, blood in your phlegm, sores on your body, warm or painful areas on your body, diarrhea or stomach pain, or feeling very tired.
See “What are possible side effects of Ilaris?” for more information about side effects.
Who should not use Ilaris?
- Do not receive Ilaris if you are allergic to canakinumab or any of the ingredients in Ilaris. See the end of this Medication Guide for a complete list of ingredients in Ilaris.
What should I tell my healthcare provider before using Ilaris?
Before you receive Ilaris, tell your healthcare provider about all your medical conditions, including if you:
- think you have or are being treated for an active infection
- have symptoms of an infection
- have a history of infections that keep coming back
- have a history of low white blood cells
- have or have had HIV, Hepatitis B, or Hepatitis C
- are scheduled to receive any immunizations (vaccines). You should not get ‘live vaccines’ if you are receiving Ilaris.
- are pregnant or planning to become pregnant. It is not known if Ilaris will harm your unborn baby. Tell your healthcare provider right away if you become pregnant while receiving Ilaris.
- received Ilaris while you were pregnant. It is important that you tell your baby’s healthcare provider before any vaccinations are given to your baby within 4-12 months after you received your last dose of Ilaris before giving birth.
- are breastfeeding or planning to breastfeed. It is not known if Ilaris passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you receive Ilaris.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take:
- medicines that affect your immune system
- medicines called IL-1 blocking agents such as Kineret (anakinra), Arcalyst (rilonacept)
- medicines called Tumor Necrosis Factor (TNF) inhibitors such as Enbrel (etanercept), Humira (adalimumab), Remicade (infliximab), Simponi (golimumab), or Cimzia (certolizumab pegol)
- medicines that effect enzyme metabolism
Ask your healthcare provider for a list of these medicines if you are not sure.
How should I use Ilaris?
- Ilaris is given by your healthcare provider every 8 weeks for CAPS and every 4 weeks for TRAPS, HIDS/MKD, FMF, and SJIA.
What are the possible side effects of Ilaris?
Ilaris can cause serious side effects, including:
- See “What is the most important information I should know about Ilaris?”
- decreased ability of your body to fight infections (immunosuppression). For people treated with medicines that cause immunosuppression like Ilaris, the chances of getting cancer may increase.
- allergic reactions. Allergic reactions can happen while you are receiving Ilaris. Call your healthcare provider right away if you have any of these symptoms of an allergic reaction:
- risk of infection with live vaccines. You should not get live vaccines if you are receiving Ilaris. Tell your healthcare provider if you are scheduled to receive any vaccines.
The most common side effects of Ilaris include:
When Ilaris is used for the treatment of CAPS:
- cold symptoms
- headache
- feeling like you are spinning (vertigo)
- diarrhea
- cough
- weight gain
- flu (influenza)
- body aches
- injection-site reactions (such as redness, swelling, warmth, or itching)
- runny nose
- nausea, vomiting, and diarrhea (gastroenteritis)
- nausea
When Ilaris is used for the treatment of TRAPS, HIDS/MKD, and FMF:
- cold symptoms
- runny nose
- nausea, vomiting, and diarrhea (gastroenteritis)
- upper respiratory tract infection
- sore throat
- Injection-site reactions (such as redness, swelling, warmth, or itching)
When Ilaris is used for the treatment of SJIA:
- cold symptoms
- runny nose
- nausea, vomiting, and diarrhea (gastroenteritis)
- upper respiratory tract infection
- sore throat
- stomach pain
- pneumonia
- urinary tract infection
- injection-site reactions (such as redness, swelling, warmth, or itching)
Tell your healthcare provider about any side effect that bothers you or does not go away.
These are not all the possible side effects of Ilaris. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Ilaris
Medicines are sometimes prescribed for purposes other than those listed in this Medication Guide. You can ask your healthcare provider or pharmacist for information about Ilaris that was written for health professionals.
How should I store Ilaris?
The unopened vial must be stored refrigerated at 2°C to 8°C (36°F to 46° F). Do not freeze. Store in the original carton to protect from light. Do not use beyond the date stamped on the label. Ilaris does not contain preservatives. Discard any unused portions of Ilaris or waste material in accordance with local requirements.
Keep this and all drugs out of the reach of children.
What are the ingredients in Ilaris?
Active ingredient: canakinumab
Inactive ingredients:
Powder for Solution for Injection: L-histidine, L-histidine HCl monohydrate, polysorbate 80, sucrose, and sterile water for injection.
Solution for Injection: L-histidine, L-histidine HCl monohydrate, mannitol, polysorbate 80, sterile water for injection.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0078-0734-61
- Rx only
- ILARIS®
(canakinumab)
Injection - 150 mg/mL
- For Subcutaneous Use
- Single-Dose Vial. Discard Unused portion
- Attention: Dispense with enclosed Medication Guide.
- NOVARTIS
SRC: NLM .
