Hemlibra
Generic name: emicizumab
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by A Ras MD.
What is Hemlibra?
Hemlibra is a prescription medicine used for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children, ages newborn and older, with hemophilia A with or without factor VIII inhibitors.
Hemophilia A is a bleeding condition people can be born with where a missing or faulty blood clotting factor (factor VIII) prevents blood from clotting normally.
Hemlibra is a therapeutic antibody that bridges clotting factors to help your blood clot.
Description
Emicizumab-kxwh is a humanized monoclonal modified immunoglobulin G4 (IgG4) bispecific antibody binding factor IXa and factor X. Emicizumab-kxwh has an approximate molecular weight of 145.6 kDa and is produced in genetically engineered mammalian (Chinese hamster ovary) cells. Emicizumab-kxwh has no structural relationship or sequence homology to FVIII and, as such, does not induce or enhance the development of direct inhibitors to FVIII.
HEMLIBRA (emicizumab-kxwh) injection is a sterile, preservative-free, colorless to slightly yellow solution for subcutaneous injection supplied in single-dose vials containing emicizumab-kxwh at 30 mg/mL, 60 mg/0.4 mL, 105 mg/0.7 mL, or 150 mg/mL.
Each single-dose 30 mg vial contains a 1 mL solution of emicizumab-kxwh (30 mg), L-arginine (26.1 mg), L-histidine (3.1 mg), and poloxamer 188 (0.5 mg), adjusted to pH 6.0 with L-aspartic acid.
Each single-dose 60 mg vial contains a 0.4 mL solution of emicizumab-kxwh (60 mg), L-arginine (10.5 mg), L-histidine (1.2 mg), and poloxamer 188 (0.2 mg), adjusted to pH 6.0 with L-aspartic acid.
Each single-dose 105 mg vial contains a 0.7 mL solution of emicizumab-kxwh (105 mg), L-arginine (18.3 mg), L-histidine (2.2 mg), and poloxamer 188 (0.4 mg), adjusted to pH 6.0 with L-aspartic acid.
Each single-dose 150 mg vial contains a 1 mL solution of emicizumab-kxwh (150 mg), L-arginine (26.1 mg), L-histidine (3.1 mg), and poloxamer 188 (0.5 mg), adjusted to pH 6.0 with L-aspartic acid.
Mechanism of Action
HEMLIBRA bridges activated factor IX and factor X to restore the function of missing activated factor VIII that is needed for effective hemostasis.
What is the most important information I should know about Hemlibra?
Hemlibra increases the potential for your blood to clot. Carefully follow your healthcare provider’s instructions regarding when to use an on-demand bypassing agent or factor VIII (FVIII) and the recommended dose and schedule to use for breakthrough bleed treatment.
Hemlibra may cause the following serious side effects when used with activated prothrombin complex concentrate (aPCC; Feiba), including:
- Thrombotic microangiopathy (TMA). This is a condition involving blood clots and injury to small blood vessels that may cause harm to your kidneys, brain, and other organs. Get medical help right away if you have any of the following signs or symptoms during or after treatment with Hemlibra:
- Blood clots (thrombotic events). Blood clots may form in blood vessels in your arm, leg, lung, or head. Get medical help right away if you have any of these signs or symptoms of blood clots during or after treatment with Hemlibra:
- swelling in arms or legs
- pain or redness in your arms or legs
- shortness of breath
- chest pain or tightness
- fast heart rate
- cough up blood
- feel faint
- headache
- numbness in your face
- eye pain or swelling
- trouble seeing
If aPCC (Feiba) is needed, talk to your healthcare provider in case you feel you need more than 100 U/kg of aPCC (Feiba) total.
See “What are the possible side effects of Hemlibra?” for more information about side effects.
What should I tell my healthcare provider before using Hemlibra?
Before using Hemlibra, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if Hemlibra may harm your unborn baby. Females who are able to become pregnant should use birth control (contraception) during treatment with Hemlibra.
- are breastfeeding or plan to breastfeed. It is not known if Hemlibra passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, or herbal supplements. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Hemlibra?
See the detailed Instructions for Use that comes with your Hemlibra for information on how to prepare and inject a dose of Hemlibra, and how to properly throw away (dispose of) used needles and syringes.
- Use Hemlibra exactly as prescribed by your healthcare provider.
- Stop (discontinue) prophylactic use of bypassing agents the day before starting Hemlibra prophylaxis.
- You may continue prophylactic use of FVIII for the first week of Hemlibra prophylaxis.
- Hemlibra is given as an injection under your skin (subcutaneous injection) by you or a caregiver.
- Your healthcare provider should show you or your caregiver how to prepare, measure, and inject your dose of Hemlibra before you inject yourself for the first time.
- Do not attempt to inject yourself or another person unless you have been taught how to do so by a healthcare provider.
- Your healthcare provider will prescribe your dose based on your weight. If your weight changes, tell your healthcare provider.
- You will receive Hemlibra 1 time a week for the first four weeks. Then you will receive a maintenance dose as prescribed by your healthcare provider.
- If you miss a dose of Hemlibra on your scheduled day, you should give the dose as soon as you remember. You must give the missed dose as soon as possible before the next scheduled dose, and then continue with your normal dosing schedule. Do not give two doses on the same day to make up for a missed dose.
- Hemlibra may interfere with laboratory tests that measure how well your blood is clotting and may cause a false reading. Talk to your healthcare provider about how this may affect your care.
What are the possible side effects of Hemlibra?
- See “What is the most important information I should know about Hemlibra?”
The most common side effects of Hemlibra include:
- redness, tenderness, warmth, or itching at the site of injection
- headache
- joint pain
These are not all of the possible side effects of Hemlibra.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Hemlibra
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Hemlibra for a condition for which it was not prescribed. Do not give Hemlibra to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Hemlibra that is written for health professionals.
How should I store Hemlibra?
- Store Hemlibra in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze.
- Store Hemlibra in the original carton to protect the vials from light.
- Do not shake Hemlibra.
- If needed, unopened vials of Hemlibra can be stored out of the refrigerator and then returned to the refrigerator. Hemlibra should not be stored out of the refrigerator for more than a total of 7 days or at a temperature greater than 86°F (30°C).
- After Hemlibra is transferred from the vial to the syringe, Hemlibra should be used right away.
- Throw away (dispose of) any unused Hemlibra left in the vial.
Keep Hemlibra and all medicines out of the reach of children.
What are the ingredients in Hemlibra?
Active ingredient: emicizumab-kxwh
Inactive ingredients: L-arginine, L-histidine, poloxamer 188, and L-aspartic acid.
Label
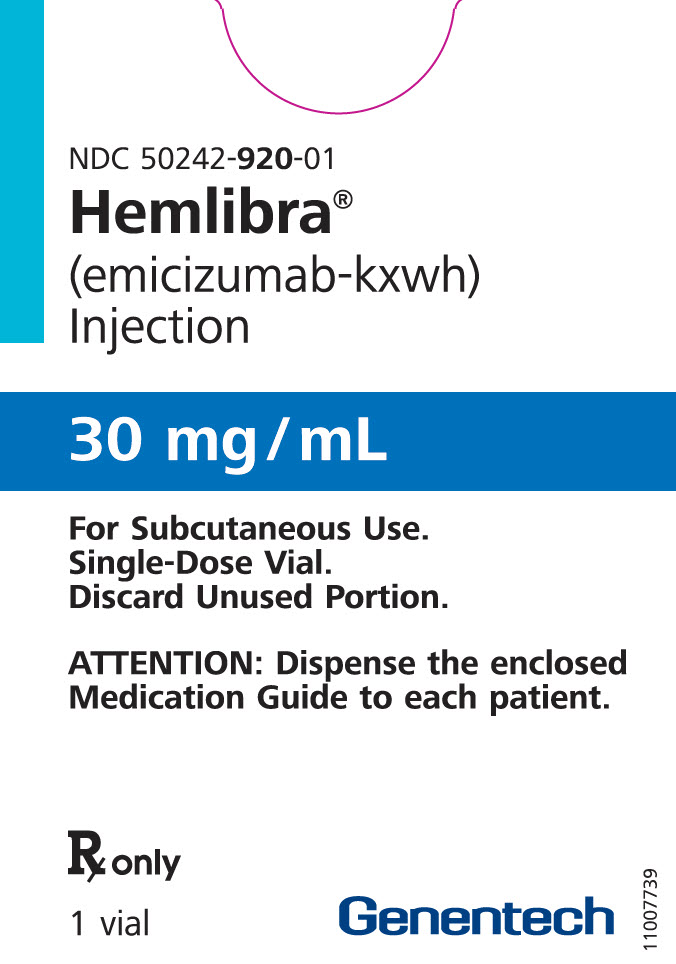
PRINCIPAL DISPLAY PANEL – 30 MG VIAL CARTON
- NDC 50242-920-01
Hemlibra®
(emicizumab-kxwh)
Injection - 30 mg/mL
- For Subcutaneous Use.
Single-Dose Vial.
Discard Unused Portion. - ATTENTION: Dispense the enclosed
Medication Guide to each patient. - Rx only
- 1 vial
Genentech - 10195119
PRINCIPAL DISPLAY PANEL – 60 MG VIAL CARTON
- NDC 50242-921-01
Hemlibra®
(emicizumab-kxwh)
Injection - 60 mg/0.4 mL
- For Subcutaneous Use.
Single-Dose Vial.
Discard Unused Portion. - ATTENTION: Dispense the enclosed
Medication Guide to each patient. - Rx only
- 1 vial
Genentech - 10195122

PRINCIPAL DISPLAY PANEL – 105 MG VIAL CARTON
- NDC 50242-922-01
Hemlibra®
(emicizumab-kxwh)
Injection - 105 mg/0.7 mL
- For Subcutaneous Use.
Single-Dose Vial.
Discard Unused Portion. - ATTENTION: Dispense the enclosed
Medication Guide to each patient. - Rx only
- 1 vial
Genentech - 10195123

PRINCIPAL DISPLAY PANEL – 150 MG VIAL CARTON
- NDC 50242-923-01
Hemlibra®
(emicizumab-kxwh)
Injection - 150 mg/mL
- For Subcutaneous Use.
Single-Dose Vial.
Discard Unused Portion. - ATTENTION: Dispense the enclosed
Medication Guide to each patient. - Rx only
- 1 vial
Genentech - 10195121

SRC: NLM .
