Gonal-f RFF Redi-ject
Generic name: follitropin alfa (subcutaneous route)
Medically reviewed by A Ras MD.
What is Gonal-f RFF Redi-ject?
Gonal-f RFF Redi-ject is an injection Pen that delivers a prescription medicine containing follicle-stimulating hormone (FSH) used in infertile women to help healthy ovaries develop (mature) and release an egg, cause your ovaries to make multiple (more than 1) eggs as part of an Assisted Reproductive Technology (ART) program
Description
Gonal-f® RFF Redi-ject® contains human follicle stimulating hormone (hFSH), a glycoprotein hormone manufactured by recombinant DNA technology. The active drug substance, follitropin alfa, has a dimeric structure consisting of two non-covalently linked, non-identical glycoproteins designated as the α-and β-subunits. The α-and β-subunits have 92 and 111 amino acids, respectively, and their primary and tertiary structures are indistinguishable from those of human follicle stimulating hormone.
Recombinant human FSH production occurs in genetically modified Chinese Hamster Ovary (CHO) cells cultured in bioreactors. Purification by immunochromatography using an antibody specifically binding FSH results in a highly purified preparation with a consistent FSH isoform profile, and a high specific activity. The protein content is assessed by size exclusion high pressure liquid chromatography.
The biological activity of follitropin alfa is determined by measuring the increase in ovary weight in female rats. The in vivo biological activity of follitropin alfa has been calibrated against the first International Standard for recombinant human follicle stimulating hormone established in 1995 by the Expert Committee on Biological Standards of the World Health Organization. Gonal-f® RFF Redi-ject® contains no luteinizing hormone (LH) activity. Based on available data derived from physico-chemical tests and bioassays, follitropin alfa and follitropin beta, another recombinant follicle stimulating hormone product, are indistinguishable.
Gonal-f® RFF Redi-ject® is a disposable, prefilled drug delivery system intended for the subcutaneous injection of multiple and variable doses of a liquid formulation of follitropin alfa.
Each Gonal-f® RFF Redi-ject® is filled with 415 International Units (30 mcg), 568 International Units (41 mcg), or 1026 International Units (75 mcg) follitropin alfa to deliver at least 300 International Units (22 mcg) in 0.5 mL, 450 International Units (33 mcg) in 0.75 mL, or 900 International Units (66 mcg) in 1.5 mL, respectively. Each Redi-ject® also contains 60 mg/mL sucrose, 3.0 mg/mL m-cresol, 1.1 mg/mL di-sodium hydrogen phosphate dihydrate, 0.45 mg/mL sodium dihydrogen phosphate monohydrate, 0.1 mg/mL methionine, 0.1 mg/mL Poloxamer 188. O-phosphoric acid and/or sodium hydroxide may be used for pH adjustment.
Under current storage conditions, Gonal-f® RFF Redi-ject® may contain up to 10% of oxidized follitropin alfa.
Therapeutic Class: Infertility
Mechanism of Action
Follicle stimulating hormone (FSH), the active component in Gonal-f® RFF Redi-ject®, is required for normal follicular growth, follicular maturation, and gonadal steroid production. The level of FSH is critical for the onset and duration of follicular development, and consequently for the timing and number of follicles reaching maturity.
Gonal-f® RFF Redi-ject® stimulates ovarian follicular growth in women who do not have primary ovarian failure. In order to effect the final phase of follicle maturation, resumption of meiosis, and rupture of the follicle in the absence of an endogenous LH surge, human chorionic gonadotropin (hCG) must be given following treatment with Gonal-f® RFF Redi-ject® when monitoring of the woman indicates that appropriate follicular development parameters have been achieved. There is inter-woman variability in response to FSH administration.
Who should not use Gonal-f RFF Redi-ject?
Do not use the Gonal-f RFF Redi-ject if you:
- are allergic to recombinant human FSH or any of the ingredients in Gonal-f RFF Redi-ject. See the end of this leaflet for a complete list of ingredients in Gonal-f RFF Redi-ject.
- have ovaries that no longer make eggs (primary ovarian failure)
- are pregnant or think you may be pregnant
- have uncontrolled thyroid or adrenal problems
- have a tumor in your female organs, including your ovaries, breast, or uterus that may get worse with high levels of estrogen
- have a tumor in your brain, such as a tumor in your pituitary or hypothalamus
- have abnormal bleeding from your uterus or vagina
- have ovarian cysts or large ovaries, not due to polycystic ovary syndrome (PCOS)
What should I tell my healthcare provider before using Gonal-f RFF Redi-ject?
Before you use Gonal-f RFF Redi-ject, tell your healthcare provider if you:
- have or have had asthma
- have been told by a healthcare provider that you have an increased risk for blood clots (thrombosis)
- have ever had a blood clot (thrombosis), or anyone in your family has ever had a blood clot (thrombosis)
- have had stomach (abdominal) surgery
- have had twisting of your ovary (ovarian torsion)
- had or have a cyst on your ovary
- have polycystic ovarian disease
- have any other medical conditions
- are breastfeeding or plan to breastfeed. It is not known if Gonal-f RFF Redi-ject passes into your breast milk. You and your healthcare provider should decide if you will take Gonal-f RFF Redi-ject or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Gonal-f RFF Redi-ject?
- Read the “Instructions for Use” that comes with Gonal-f RFF Redi-ject for information about the right way to use Gonal-f RFF Redi-ject.
- Gonal-f RFF Redi-ject is given by injection under your skin. Do not inject Gonal-f RFF Redi-ject until your healthcare provider has taught you the correct way to use it.
- Change your injection site as your healthcare provider showed you.
- Use Gonal-f RFF Redi-ject exactly as your healthcare provider tells you to use it.
- Do not change your dose of Gonal-f RFF Redi-ject unless your healthcare provider tells you to.
- Call your healthcare provider if you have any questions about your dose or how to use Gonal-f RFF Redi-ject.
What are the possible side effects of Gonal-f RFF Redi-ject?
Gonal-f RFF Redi-ject may cause serious side effects, including:
- severe allergic reactions. Women who have used Gonal-f, Gonal-f RFF, or Gonal-f RFF Redi-ject in the past may have a severe allergic reaction right away when they use Gonal-f RFF Redi-ject again. This severe allergic reaction may lead to death. If you have any of the following symptoms of a severe allergic reaction, stop using Gonal-f RFF Redi-ject and go to the hospital right away:
- shortness of breath
- swelling of your face
- itchy, red bumps or rash on your skin (hives)
- ovaries that are too large. Gonal-f RFF Redi-ject may cause your ovaries to be abnormally large. Symptoms of large ovaries include bloating or pain in your lower stomach (pelvic) area.
- ovarian hyperstimulation syndrome (OHSS). Using Gonal-f RFF Redi-ject may cause OHSS. OHSS is a serious medical condition that can happen when your ovaries produce too many eggs (overstimulated). OHSS can cause fluid to suddenly build up in the area of your stomach, chest, and heart, and can cause blood clots to form. In rare cases OHSS has caused death. OHSS may also happen after you stop using Gonal-f RFF Redi-ject. Stop using Gonal-f RFF Redi-ject and call your healthcare provider right away if you have symptoms of OHSS, including:
- lung problems. Gonal-f RFF Redi-ject may cause serious lung problems including fluid in your lungs (atelectasis), trouble breathing (acute respiratory distress syndrome), and worsening of asthma.
- blood clots. Gonal-f RFF Redi-ject may increase your chance of having blood clots in your blood vessels. Blood clots can cause:
- blood vessel problems (thrombophlebitis)
- stroke
- loss of your arm or leg
- blood clot in your lung (pulmonary embolus)
- twisting (torsion) of your ovary. Gonal-f RFF Redi-ject may increase the chance of your ovary twisting if you already have certain conditions such as OHSS, pregnancy and previous abdominal surgery. Twisting of your ovary may lead to blood flow being cut off to your ovary.
- pregnancy with and birth of multiple babies. Gonal-f RFF Redi-ject may increase your chance of having a pregnancy with more than 1 baby. Having a pregnancy and giving birth to more than 1 baby at a time increases the health risk for you and your babies. Your healthcare provider should tell you about your chances of multiple births.
- birth defects. A baby born after an ART cycle may have an increased chance of having birth defects. Your chances of having a baby with birth defects may increase depending on:
- your age
- certain sperm problems
- your genetic background and that of your partner
- a pregnancy with more than 1 baby at a time
- ectopic pregnancy (pregnancy outside your womb). Gonal-f RFF Redi-ject may increase your chance of having a pregnancy that is abnormally outside of your womb. Your chance of having a pregnancy outside of your womb is increased if you also have fallopian tube problems.
- miscarriage. Your chance of loss of an early pregnancy may be increased if you had difficulty becoming pregnant.
- tumors of the ovary. If you have used medicines like Gonal-f RFF Redi-ject more than 1 time to get pregnant, you may have an increased chance of having tumors in your ovary(ies) (including cancer).
The most common side effects of Gonal-f RFF Redi-ject include:
- headache
- stomach pain
- stomach bloating
- bruising at the injection site
- nausea
These are not all the possible side effects of Gonal-f RFF Redi-ject. For more information, call your healthcare provider or pharmacist.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Gonal-f RFF Redi-ject
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Gonal-f RFF Redi-ject for a condition for which it was not prescribed. Do not give Gonal-f RFF Redi-ject to other people, even if they have the same condition that you have. It may harm them.
This Patient Information leaflet summarizes the most important patient information about Gonal-f RFF Redi-ject. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Gonal-f RFF Redi-ject that is written for health professionals.
For more information, go to www.fertilitylifelines.com, or call 1-866-538-7879.
How should I store Gonal-f RFF Redi-ject?
- Before you use Gonal-f RFF Redi-ject for the first time, store your Pen:
- in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date, or
- store your Pen at room temperature between 68°F to 77°F (20°C to 25°C) for up to 3 months or until the expiration date, whichever comes first
- After you use Gonal-f RFF Redi-ject and there is still medicine left, store your Pen in the refrigerator between 36°F to 46°F (2°C to 8°C) or at room temperature between 68°F to 77°F (20°C to 25°C) up to 28 days. Throw away any unused Gonal-f RFF Redi-ject after 28 days.
- Store your Gonal-f RFF Redi-ject with the Pen cap on in a safe place.
- Store Gonal-f RFF Redi-ject away from light.
- Do not freeze Gonal-f RFF Redi-ject.
Keep Gonal-f RFF Redi-ject and all medicines out of the reach of children.
What are the ingredients in Gonal-f RFF Redi-ject?
Active ingredient: follitropin alfa (r-hFSH)
Inactive ingredients: sucrose, meta-cresol, di-sodium hydrogen phosphate dihydrate, sodium dihydrogen phosphate monohydrate, methionine, Poloxamer 188, O-phosphoric acid, sodium hydroxide
Label
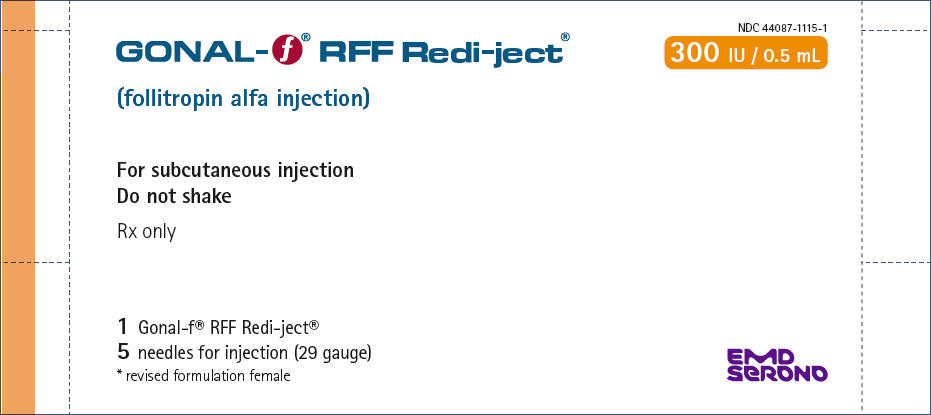
PRINCIPAL DISPLAY PANEL – 300 IU KIT CARTON
- NDC 44087-1115-1
- GONAL-f ® RFF Redi-ject®
- 300 IU / 0.5 mL
- (follitropin alfa injection)
- For subcutaneous injection
Do not shake - Rx only
- 1 Gonal-f® RFF Redi-ject®
5 needles for injection (29 gauge)
* revised formulation female - EMD
SERONO
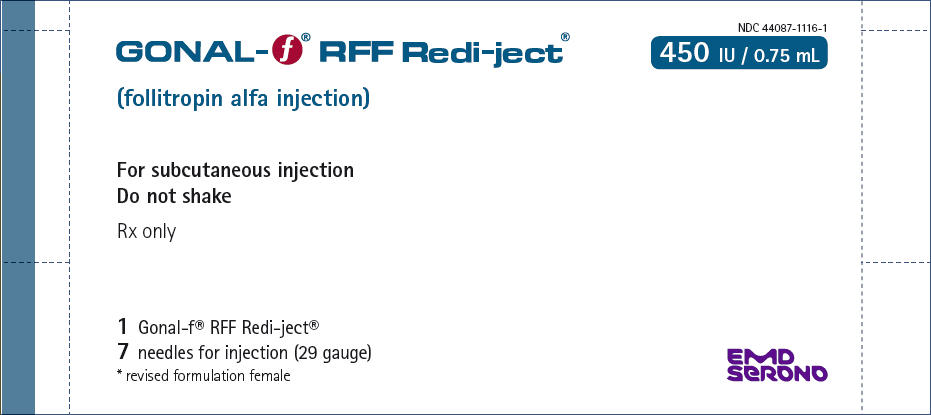
PRINCIPAL DISPLAY PANEL – 450 IU KIT CARTON
- NDC 44087-1116-1
- GONAL-f ® RFF Redi-ject®
- 450 IU / 0.75 mL
- (follitropin alfa injection)
- For subcutaneous injection
Do not shake - Rx only
- 1 Gonal-f® RFF Redi-ject®
7 needles for injection (29 gauge)
* revised formulation female - EMD
SERONO