Gonal-f
Generic name: follicle stimulating hormone
Brand names: Follistim AQ Cartridge, Gonal-F, Gonal-f RFF
Drug class: Gonadotropins
Medically reviewed by A Ras MD.
What is Gonal-f?
Gonal-f Multi-Dose is an injectable hormone contained in a stoppered glass vial. The hormone in the vial is in the form of a white powder. The carton containing the vial of drug also contains a syringe labeled ‘Bacteriostatic Water for Injection, USP’. This water must be mixed with the white powder in the vial to form a clear liquid solution for injection. Injection syringes for use with Gonal-f Multi-Dose are also included in the carton. These injection syringes can only be used to administer Gonal-f Multi-Dose. Gonal-f Multi-Dose is only available with a prescription.
Gonal-f Multi-Dose contains follitropin alfa, which is similar to the human hormone ‘follicle stimulating hormone’; the abbreviation is ‘FSH’. FSH belongs to the group of hormones associated with human reproduction. In women, FSH causes the ovaries to produce eggs. In men, FSH causes sperm production.
The hormone in Gonal-f Multi-Dose is manufactured to meet standards for quality and purity. It cannot be taken by mouth since the acids in your stomach would destroy the hormone before it was absorbed into the body. Gonal-f Multi-Dose is given as an injection usually every day in women and three times per week in men. It is prescribed to patients needing hormone replacement or supplementation to produce either eggs or sperm.
The Gonal-f Multi-Dose 450 IU (33 mcg) vial is filled with 600 IU of drug in order to deliver 450 IU in several smaller daily doses. This provides between 2 and 6 commonly prescribed daily doses.
The Gonal-f Multi-Dose 1050 IU (77 mcg) vial is filled with 1200 IU of drug in order to deliver 1050 IU in several smaller daily doses. This provides between 3 and 14 commonly prescribed daily doses.
Your doctor or nurse will tell you the number of units (IU FSH) of Gonal-f to use each day and the number of days to use the same vial. It is common for a small amount of drug to be leftover in each vial that can not be retrieved with a syringe. This is normal. Any drug remaining in the vial after your treatment is complete should be discarded.
Your doctor, nurse, or pharmacist will show you how to inject the prescribed dose. Usual injection sites include the skin on the stomach, upper leg, or upper arm.
Description
GONAL-F (follitropin alpha for injection) is a gonadotropin [human follicle stimulating hormone (FSH)], a glycoprotein hormone manufactured by recombinant DNA technology. The active drug substance, follitropin alfa, has a dimeric structure consisting of two non-covalently linked, non-identical glycoproteins designated as the α- and β-subunits. The α- and β-subunits have 92 and 111 amino acids, respectively, and their primary and tertiary structures are indistinguishable from those of human follicle stimulating hormone.
Recombinant FSH production occurs in genetically modified Chinese Hamster Ovary (CHO) cells cultured in bioreactors. Purification by immunochromatography using an antibody specifically binding FSH results in a highly purified preparation with a consistent FSH isoform profile, and a high specific activity. The protein content is assessed by size exclusion high pressure liquid chromatography. The biological activity of follitropin alfa is determined by measuring the increase in ovary weight in female rats. The in vivo biological activity of follitropin alfa has been calibrated against the first International Standard for Recombinant Human Follicle Stimulation Hormone established in 1995 by the Expert Committee on Biological Standards of the World Health Organization. GONAL-F contains no luteinizing hormone (LH) activity. Based on available data derived from physico-chemical tests and bioassays, follitropin alfa and follitropin beta, another recombinant follicle stimulating hormone product, are indistinguishable.
GONAL-F is a sterile, lyophilized powder intended for subcutaneous injection after reconstitution.
Each GONAL-F Multi-Dose vial is filled with 600 International Units (44 mcg) or 1200 International Units (87 mcg) follitropin alfa to deliver 450 International Units (33 mcg) or 1050 International Units (77 mcg) follitropin alfa, respectively, and contains 30 mg sucrose, 1.11 mg dibasic sodium phosphate dihydrate and 0.45 mg monobasic sodium phosphate monohydrate. O-phosphoric acid and/or sodium hydroxide may be used prior to lyophilization for pH adjustment. Multiple Dose vials are reconstituted with Bacteriostatic Water for Injection (0.9% benzyl alcohol), USP.
Under current storage conditions, GONAL-F may contain up to 10% of oxidized follitropin alfa.
Mechanism of Action
GONAL-F stimulates ovarian follicular growth in women who do not have primary ovarian failure. In order to bring about final maturation of the follicle and ovulation in the absence of an endogenous LH surge, human chorionic gonadotropin (hCG) must be given, following the administration of GONAL-F, when monitoring of the patient indicates that sufficient follicular development is achieved.
GONAL-F stimulates spermatogenesis in men with hypogonadotropic hypogonadism when administered with hCG.
What are the uses of Gonal-f Multi-Dose?
Doctors specializing in infertility or reproductive health prescribe Gonal-f Multi-Dose to those patients trying to have a child but for a variety of reasons need medical assistance. After a thorough medical exam to determine your specific medical condition, your doctor may prescribe Gonal-f Multi- Dose because you require hormone replacement or supplementation as part of your treatment program. Gonal-f Multi-Dose can be used in women seeking pregnancy or in men with a rare condition that affects sperm production. Gonal-f Multi-Dose may be one of several drugs prescribed to a patient as part of a treatment program.
What is the most important information I should know about Gonal-F?
The Gonal-f liquid solution may be stored refrigerated or at room temperature for a maximum of 28 days from the day the powder is mixed with the water. Do Not Freeze. Discard unused liquid solution after 28 days.
Use only the prescribed dose. Call your doctor immediately should you accidentally inject more than the prescribed dose.
Who should not take Gonal-f?
Do not take Gonal-f Multi-Dose if you have allergies to any of these materials:
- follitropin
- sucrose
- sodium phosphate
- benzyl alcohol
Do not take Gonal-f Multi-Dose if you are pregnant or breast feeding.
What should I tell my healthcare provider before taking Gonal-f?
Medical conditions you should tell your doctor about.
If you have any of the following conditions, make sure to tell your doctor before starting or continuing use of Gonal-f:
- Abnormal bleeding from the uterus or vagina in women
- Swollen, enlarged or painful ovaries in women
- Cancer of the sex organs (uterus, ovaries, testes)
- Permanent damage to the male sex organs (testes)
- Uncontrolled thyroid or adrenal problems
- Cancer of the brain
How should I take Gonal-f?
See your doctor, nurse, or pharmacist to obtain training in the preparation and use of Gonal-f Multi-Dose.
Review the steps below in the Instructions for Use before you prepare or administer Gonal-F Multi-Dose.
What should you do if you forget to take Gonal-f Multi-Dose?
Do not take a double dose of Gonal-f. Contact your doctor if you forget to take a dose of Gonal-f.
Can you take Gonal-f Multi-Dose with other medicines?
Inform your doctor and pharmacist if you are taking or have taken any other medicines, even those not requiring a prescription.
What are the possible side effects of Gonal-f?
Your doctor or staff member should review with you the risks and benefits of using Gonal-f Multi-Dose. As with any medication, report any and all side effects, symptoms, or physical changes to your doctor.
The most common side effects are headache, ovarian cysts, upset stomach, and sinus infections in women and skin pimples, breast pain and growth, and tiredness in men. Needle injections may cause some discomfort.
Use of fertility drugs can be associated with fertilization of more than 1 egg. This can lead to complications for the mother and the birth of 2 or more babies. Pregnancy loss (miscarriage) is higher in women receiving fertility drugs than in women not taking fertility drugs.
Gonal-f is a potent drug which should be used at the lowest dose expected to achieve the desired results. When used in women, your doctor should monitor your response often to avoid overdose which can lead to serious side effects including blood clots.
IMPORTANT
| Contact your doctor if you take more than the prescribed amount of Gonal-f or experience severe pain or bloating in the stomach or pelvic area, severe upset stomach, vomiting, and weight gain. |
In rare cases, ovarian cancer has been reported in women receiving many courses of fertility drugs.
General information about the safe and effective use of Gonal-f
This leaflet is a summary of the important patient information about Gonal-f Multi-Dose. If you have any questions or problems, talk to your doctor or other health care provider.
Gonal-f Multi-Dose is manufactured and distributed by EMD Serono, Inc. You can also visit the Web site www.fertilitylifelines.com or contact EMD Serono at 1-866-538-7879.
How should I store Gonal-f?
- After each use, the vial containing the Gonal-f Multi-Dose liquid must be stored away from light and may be stored refrigerated or at room temperature between 36°- 77° F (2°- 25° C) for up to 28 days. Otherwise, the drug’s potency can be reduced. Do not store the drug in the syringe.
- If you are traveling, keep the vial stored away from light and extreme temperatures. Do not freeze.
- Allow the liquid solution to adjust to room temperature prior to administering your injection.
- Check that the Gonal-f liquid solution is clear. Do not use if it contains any particles. Report this to your doctor, nurse or pharmacist immediately.
What are the ingredients in Gonal-f?
Active ingredient: follitropin
Inactive ingredients: sucrose; sodium phosphate, dibasic, dihydrate; sodium phosphate, monobasic, monohydrate; phosphoric acid; sodium hydroxide; water; benzyl alcohol.
Instructions for use
How to Prepare Gonal-f Multi-Dose for Use
See your doctor, nurse, or pharmacist to obtain training in the preparation and use of Gonal-f Multi-Dose.
| Review these steps before you prepare or administer Gonal-F Multi-Dose |
Getting ready
Make sure you have all the necessary items listed below before you begin.
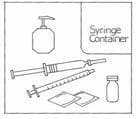
- vial containing Gonal-f Multi-Dose (white powder)
- single pre-filled syringe labeled ‘Bacteriostatic Water for Injection, USP’
- 27-gauge injection syringe with unit dose markings from 37.5 IU to 600 IU FSH for use with the Gonal-f Multi-Dose.
- alcohol wipes
- hard plastic or metal container (like an empty coffee can) suitable for safe disposal of used syringes and needles.
Step 1: Mixing (reconstituting) the vial containing Gonal-f Multi-Dose
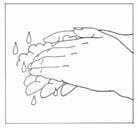
- Wash your hands with soap and water.
- Using your thumb, flip off the plastic cap of the Gonal-f Multi-Dose vial.
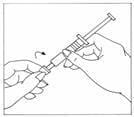
- Wipe the top of the vial stopper with an alcohol wipe.
- Carefully twist the needle cap off the syringe labeled ‘Bacteriostatic Water for Injection, USP’. Do not touch the needle or allow the needle to touch any surface.
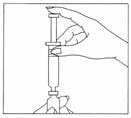
- Position the needle of the syringe of water in a straight, upright position over the marked center circle of the rubber stopper on the vial of Gonal-f Multi-Dose powder. Keep the needle in a straight, upright position as you insert it through the center circle, or it may be difficult to depress the plunger. Slowly inject the water into the vial by depressing the syringe plunger. The water and white powder will mix to form a clear liquid. When all the water has been injected into the vial, withdraw the needle and safely dispose of it immediately in your needle container. Do not use this needle to inject your dose.
- Do not shake the vial. If bubbles appear, wait a few moments for the bubbles to settle. The liquid drug should be clear.
IMPORTANT
| Do not use the Gonal-f Multi-Dose liquid solution if it contains any particles. Report this to your doctor, nurse, or pharmacist immediately. |
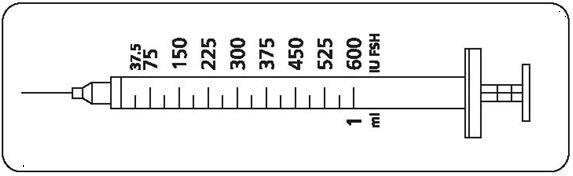
Step 2: Determining your dose on the injection syringe
Your doctor will instruct you to take a specific dose of Gonal-f Multi-Dose. Your doctor, nurse, or pharmacist should show you how to locate the syringe marking that corresponds to your prescribed dose (see illustration below).
IMPORTANT
| If your doctor or nurse instructs you to increase or decrease your dose for 1 or more days, find the correct dose marking on the injection syringe and make the change as directed. Contact your doctor or nurse if you have questions. |
Step 3: Preparing your dose
- Wipe the rubber stopper of the vial of Gonal-f Multi-Dose liquid with an alcohol wipe.
- Carefully pull the cap from the needle. Do not touch the needle or allow the needle to touch any surface. Firmly hold the vial of Gonal-f Multi-Dose liquid on a flat surface, insert the needle through the marked center circle of the rubber stopper.
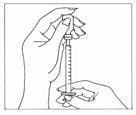
- Keeping the needle in the vial, lift the vial and turn it upside down with the needle pointing toward the ceiling. With the needle tip in the liquid, slowly pull back the plunger until the syringe fills to slightly more than the mark for your prescribed dose. Next, keeping the needle in the vial, slowly adjust the plunger to your prescribed dose – this will clear away any air bubbles.
- Check that you have the plunger set at your prescribed dose.
- Remove the syringe needle from the vial. Do not touch the needle or allow the needle to touch any surface.
You should now be ready to prepare to receive the injection.
Step 4: Injecting your dose
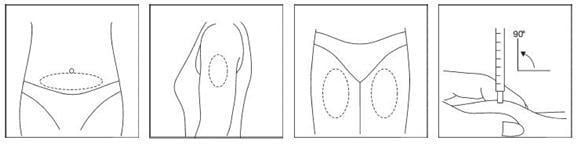
Your doctor, nurse, or pharmacist should provide you with injection training. Inject the prescribed dose as directed. Usual injection sites include the skin on the stomach, upper arm, or upper leg. Change the injection location each day to minimize discomfort. Dispose of all used syringes and needles safely in a container.
IMPORTANT
| The injection syringes provided with Gonal-f Multi-Dose are designed for use only with this product. Do NOT use the injection syringes to administer other drugs or hormones. All unused syringes should be discarded. |
Step 5: Storing Your Vial of Gonal-f Multi-Dose Between Uses
- After each use, the vial containing the Gonal-f Multi-Dose liquid must be stored away from light and may be stored refrigerated or at room temperature between 36°- 77° F (2°- 25° C) for up to 28 days. Otherwise, the drug’s potency can be reduced. Do not store the drug in the syringe.
- If you are traveling, keep the vial stored away from light and extreme temperatures. Do not freeze.
- Allow the liquid solution to adjust to room temperature prior to administering your injection.
- Check that the Gonal-f liquid solution is clear. Do not use if it contains any particles. Report this to your doctor, nurse or pharmacist immediately.
Label
PRINCIPAL DISPLAY PANEL – KIT CARTON – 450 IU
- NDC 44087-9030-1
- GONAL-f® Multi-Dose 450 IU
(follitropin alfa for injection) - For subcutaneous injection
Rx only - 1 vial GONAL-f® Multi-Dose
1 pre-filled syringe of Bacteriostatic Water
for Injection, USP (0.9% benzyl alcohol)
6 administration syringes with fixed needle
(27-gauge) - EMD
SERONO

PRINCIPAL DISPLAY PANEL – KIT CARTON – 1050 IU
- NDC 44087-9070-1
- GONAL-f® Multi-Dose 1050 IU
(follitropin alfa for injection) - For subcutaneous injection
Rx only - 1 vial GONAL-f® Multi-Dose
1 pre-filled syringe of Bacteriostatic Water
for Injection, USP (0.9% benzyl alcohol)
10 administration syringes with fixed needle
(27-gauge) - EMD
SERONO

SRC: NLM .
