Fycompa
Generic name: perampanel
Drug class: AMPA receptor antagonists
Medically reviewed by A Ras MD.
What is Fycompa?
Fycompa is a prescription medicine used to treat partial-onset seizures with or without secondarily generalized seizures in people with epilepsy who are 4 years of age and older with other medicines to treat primary generalized tonic-clonic seizures in people with epilepsy who are 12 years of age and older
Fycompa is a controlled substance (CIII) because it can be abused or lead to drug dependence. Keep your Fycompa in a safe place to protect it from theft. Never give your Fycompa to anyone else because it may harm them. Selling or giving away this medicine is against the law.
It is not known if Fycompa is safe and effective for partial onset seizures in children under 4 years of age or for primary generalized tonic clonic seizures in patients under 12 years of age.
Description
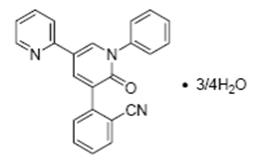
FYCOMPA tablets and oral suspension contain perampanel, a non-competitive AMPA receptor antagonist, as a 4:3 hydrate.
The chemical name of the active ingredient is 2-(1′,6′-dihydro-6′-oxo-1′-phenyl[2,3′-bipyridin]-5′-yl)-benzonitrile, hydrate (4:3).
The molecular formula is C23H15N3O •¾H2O and the molecular weight is 362.90 (349.39 for anhydrous perampanel). It is a white to yellowish white powder. It is freely soluble in 1-methyl-2-pyrrolidinone, sparingly soluble in acetonitrile and acetone, slightly soluble in methanol, ethanol and ethyl acetate, very slightly soluble in 1-octanol and diethyl ether, and practically insoluble in heptane and water. The chemical structure is:
Tablets
FYCOMPA tablets are round, bi-convex, film-coated tablets containing 2 mg, 4 mg, 6 mg, 8 mg, 10 mg, or 12 mg of perampanel. Tablets contain the following inactive ingredients: lactose monohydrate, low substituted hydroxypropyl cellulose, povidone, microcrystalline cellulose, magnesium stearate, hypromellose, polyethylene glycol, talc, and titanium dioxide. Tablets of different strengths may contain yellow ferric oxide (10 mg and 2 mg), red ferric oxide (2 mg, 4 mg, 6 mg, 8 mg), black ferric oxide (8 mg), and FD&C Blue No. 2 (indigo carmine) aluminum lake (10 mg and 12 mg).
Oral Suspension
FYCOMPA oral suspension is a white to off-white opaque liquid providing perampanel in a concentration of 0.5 mg/mL. The oral suspension contains the following inactive ingredients: sorbitol, microcrystalline cellulose, carboxymethyl-cellulose sodium, poloxamer, simethicone, citric acid, sodium benzoate and purified water.
Mechanism of Action
Perampanel is a non-competitive antagonist of the ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor on post-synaptic neurons. Glutamate is the primary excitatory neurotransmitter in the central nervous system and is implicated in a number of neurological disorders caused by neuronal over excitation.
The precise mechanism by which FYCOMPA exerts its antiepileptic effects in humans is unknown.
What is the most important information I should know about Fycompa?
1. Fycompa may cause mental (psychiatric) problems, including:
- new or worse aggressive behavior (including homicidal behavior), hostility, anger, anxiety, or irritability
- being suspicious or distrustful (believing things that are not true)
- seeing objects or hearing things that are not there
- confusion
- difficulty with memory
- other unusual or extreme changes in behavior or mood
Tell your healthcare provider right away if you have any new or worsening mental problems while taking Fycompa.
2. Like other antiepileptic drugs, Fycompa may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- new or worse depression
- feeling agitated or restless
- trouble sleeping (insomnia)
- acting aggressive, being angry, or violent
- an extreme increase in activity and talking (mania)
- attempt to commit suicide
- new or worse anxiety
- panic attacks
- new or worse irritability
- acting on dangerous impulses
- other unusual changes in behavior or mood
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop Fycompa without first talking with a healthcare provider. Stopping Fycompa suddenly can cause serious problems. Stopping Fycompa suddenly can cause you to have seizures more often.
What should I tell my healthcare provider before taking Fycompa?
Before taking Fycompa, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had depression, mood problems, aggressive or hostile behavior (for example, homicidal behavior), suicidal thoughts or behavior, or other psychiatric problems.
- have liver or kidney problems
- drink alcohol
- have abused prescription medicines, street drugs, or alcohol in the past
- are pregnant or plan to become pregnant. It is not known if Fycompa will harm your unborn baby.
- If you become pregnant while taking Fycompa, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334 or go to www.aedpregnancyregistry.org. The purpose of this registry is to collect information about the safety of Fycompa and other antiepileptic medicine during pregnancy.
- are breastfeeding or plan to breastfeed. It is not known if Fycompa passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take Fycompa. You and your healthcare provider should decide if you will take Fycompa or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking Fycompa with certain other medicines can cause side effects or reduce the benefit of either drug. Especially tell your healthcare provider if you take:
- contraceptives (birth control). Fycompa may lower your contraceptive’s ability to prevent pregnancy if your contraceptive contains levonorgestrel. Use an additional non-hormonal form of contraception (like condoms or a diaphragm and spermicide) while using Fycompa and for 1 month after you have stopped taking Fycompa.
- carbamazepine (Carbatrol, Tegretol, Tegretol-Xr, Equetro, Epitol)
- phenytoin (Dilantin, Phenytek)
- oxcarbazepine (Trileptal)
- rifampin (Rifadin, Rimactane)
- St. John’s Wort
How should I take Fycompa?
- See the Instructions for Use that come with Fycompa for complete information on how to use the dosing syringe and how to measure your dose of Fycompa Oral Suspension.
- Take Fycompa exactly as your healthcare provider tells you. Your healthcare provider will tell you how much Fycompa to take and when to take it. Fycompa is usually taken 1 time a day at bedtime.
- Your healthcare provider may change your dose. Do not change your dose without talking to your healthcare provider.
- If you take Fycompa Oral Suspension, shake the bottle well before you take each dose.
- Measure your dose of Fycompa Oral Suspension using the bottle adapter and dosing syringe provided. Do not use a household teaspoon.
- Talk to your healthcare provider about what to do if you miss 1 or more doses of Fycompa.
- If you take too much Fycompa, call your local Poison Control Center or go to the nearest hospital emergency room right away.
What should I avoid while taking Fycompa?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how Fycompa affects you. Fycompa may make you dizzy, sleepy, or tired.
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking Fycompa until you talk to your healthcare provider. Fycompa taken with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse. Fycompa when taken with alcohol may also make your mood worse, increase anger, confusion, and depression.
What are the possible side effects of Fycompa?
See “What is the most important information I should know about Fycompa?”
Fycompa may cause other serious side effects, including:
- Dizziness, vertigo (sense of spinning), and problems walking normally. You may have problems walking normally if you are unsteady because you feel dizzy. These symptoms can increase when your dose of Fycompa is increased. Your risk of feeling dizzy and having problems walking normally may be higher if you are elderly.
- Sleepiness and tiredness. See “What should I avoid while taking Fycompa?”
- Increased risk of falls. Taking Fycompa can increase your chance of falling. These falls can cause serious injuries. Your risk of falling may be higher if you are elderly.
- A serious allergic reaction that may affect your skin or other parts of your body such as your liver, kidneys, heart, or blood cells. This allergic reaction can be life-threatening and can cause death. Call your healthcare provider right away if you have:
- a skin rash, hives
- fever or swollen glands that do not go away
- swelling of your face
- shortness of breath, swelling of the legs, yellowing of the skin or whites of the eyes, or dark urine.
The most common side effects of Fycompa include:
- dizziness
- sleepiness
- tiredness
- irritability
- falls
- nausea and vomiting
- weight gain
- vertigo (sense of spinning)
- problems walking normally
- problems with muscle coordination
- headache
- bruising
- abdominal pain
- anxiety
These are not all of the possible side effects of Fycompa. For more information ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Fycompa
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Fycompa for a condition for which it was not prescribed. Do not give Fycompa to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Fycompa that is written for health professionals.
How should I store Fycompa?
- Store Fycompa tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Fycompa oral suspension below 86°F (30°C). Do not freeze.
- Replace the cap tightly after opening.
- Use Fycompa oral suspension within 90 days after the bottle is first opened.
Keep Fycompa and all medicines out of the reach of children.
What are the ingredients in Fycompa?
Active ingredient: perampanel
Inactive ingredients
Tablets: lactose monohydrate, low substituted hydroxypropyl cellulose, povidone, microcrystalline cellulose, magnesium stearate, hypromellose, polyethylene glycol, talc, and titanium dioxide. Tablets of different strengths also may contain yellow ferric oxide (10 mg and 2 mg), red ferric oxide (2 mg, 4 mg, 6 mg, 8 mg), black ferric oxide (8 mg), and FD&C blue # 2 (indigo carmine) aluminum lake (10 mg and 12 mg).
Oral suspension: sorbitol, microcrystalline cellulose, carboxymethylcellulose sodium, poloxamer, simethicone, citric acid, sodium benzoate, and purified water.
Label
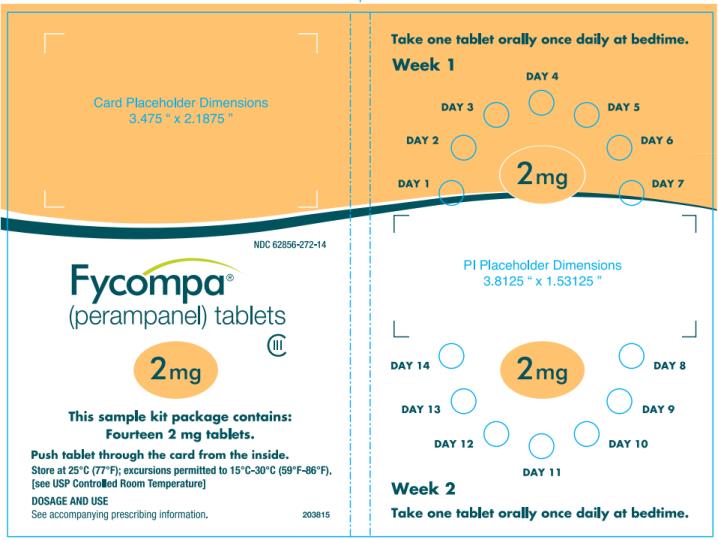
PRINCIPAL DISPLAY PANEL – 2 mg Tablet
- NDC 62856-272-14
- 14 tablets
- Rx only
- Fycompa
(perampanel) tablets - CIII
- 2 mg
- 2 week sample Kit
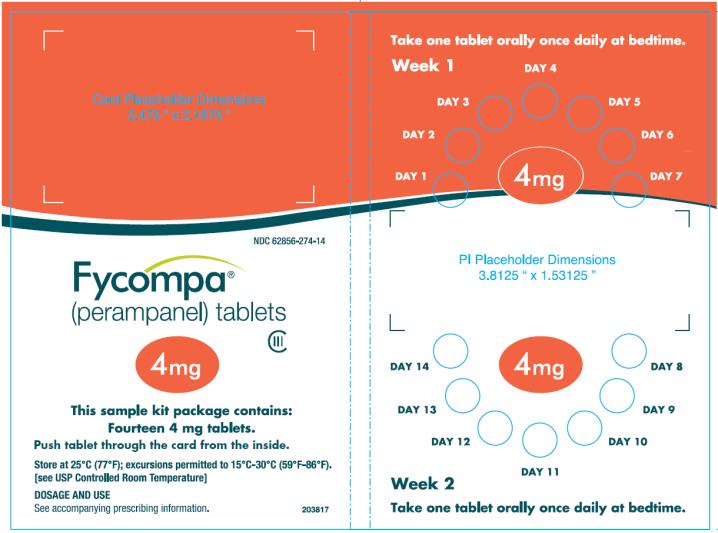
PRINCIPAL DISPLAY PANEL – 4 mg Tablet
- NDC 62856-274-14
- 14 tablets
- Rx only
- Fycompa
(perampanel) tablets - CIII
- 4 mg
- 2 week sample Kit
PRINCIPAL DISPLAY PANEL – 6 mg Tablet
- NDC 62856-276-30
- 30 tablets
- Rx only
- Fycompa™
(perampanel)
tablets - CIII
- 6 mg
- ATTENTION PHARMACIST:
Dispense the accompanying
Medication Guide to each patient.
SRC: NLM .