Fotivda
Generic name: tivozanib
Dosage form: Capsules
Drug class: VEGF/VEGFR inhibitors
Medically reviewed by A Ras MD.
What is Fotivda?
Fotivda is a prescription medicine used to treat adults with advanced kidney cancer (advanced renal cell carcinoma or RCC) that has been treated with 2 or more prior medicines and has come back or did not respond to treatment.
It is not known if Fotivda is safe and effective in children.
Description
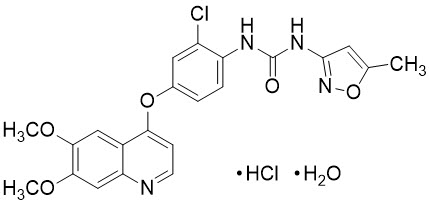
Tivozanib is a kinase inhibitor. Tivozanib hydrochloride, the active ingredient, has the chemical name 1-{2-chloro-4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-3-(5-methylisoxazol-3-yl)urea hydrochloride hydrate. The molecular formula is C22H19ClN4O5 ∙ HCl ∙ H2O and the molecular weight is 509.34 Daltons. The chemical structure is:
Tivozanib hydrochloride is a white to light brown crystalline powder that is practically insoluble in water (0.09 mg/mL).
FOTIVDA 1.34 mg capsule contains 1.5 mg of tivozanib hydrochloride (equivalent to 1.34 mg tivozanib) with inactive ingredients: mannitol and magnesium stearate. Capsule composition: gelatin, titanium dioxide, FDA yellow iron oxide, and Blue SB-6018 (ink).
FOTIVDA 0.89 mg capsule contains 1.0 mg of tivozanib hydrochloride (equivalent to 0.89 mg tivozanib) with inactive ingredients: mannitol and magnesium stearate. Capsule composition: gelatin, titanium dioxide, FDA yellow iron oxide, FD&C Blue #2, Blue SB-6018 (ink) and Yellow SB-3017 (ink). The Yellow SB-3017 ink contains FD&C Yellow No.5 (tartrazine).
Mechanism of Action
Tivozanib is a tyrosine kinase inhibitor. In vitro cellular kinase assays demonstrated that tivozanib inhibits phosphorylation of vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2 and VEGFR-3 and inhibits other kinases including c-kit and PDGFR β at clinically relevant concentrations. In tumor xenograft models in mice and rats, tivozanib inhibited angiogenesis, vascular permeability, and tumor growth of various tumor cell types including human renal cell carcinoma.
What should I tell my healthcare provider before taking Fotivda?
Before taking Fotivda, tell your healthcare provider about all your medical conditions, including if you:
- have high blood pressure.
- have a history of heart failure.
- have a history of blood clots in your veins or arteries (types of blood vessels), including stroke, heart attack, or change in vision.
- have bleeding problems.
- have thyroid problems.
- have liver problems.
- have an unhealed wound.
- plan to have surgery or have had recent surgery, including dental surgery. You should stop taking Fotivda at least 24 days before planned surgery. See “What are the possible side effects of Fotivda?”
- are allergic to FD&C Yellow No.5 (tartrazine) or aspirin.
- are pregnant or plan to become pregnant. Fotivda can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with Fotivda.
- Use effective birth control (contraception) during treatment and for 1 month after your last dose of Fotivda. Talk to your healthcare provider about birth control methods that may be right for you during this time.
- Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with Fotivda.
Males with female partners who are able to become pregnant:
- Use effective birth control (contraception) during treatment and for 1 month after your last dose of Fotivda.
- If your female partner becomes pregnant during your treatment with Fotivda, tell your healthcare provider right away.
- are breastfeeding or plan to breastfeed. It is not known if Fotivda passes into your breast milk. Do not breastfeed during treatment and for 1 month after your last dose of Fotivda.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking Fotivda with certain other medicines may affect how Fotivda works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Fotivda?
- Take Fotivda exactly as your healthcare provider tells you to take it.
- Take Fotivda 1 time each day for 21 days on treatment, followed by 7 days off treatment. This is 1 cycle of treatment. You will repeat this cycle for as long as your healthcare provider tells you to.
- Fotivda can be taken with or without food.
- Swallow the Fotivda capsule whole with a glass of water. Do not open the capsule.
- If you miss a dose of Fotivda, take your next dose at your next scheduled time. Do not take 2 doses in the same day.
- If you take too much Fotivda, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Fotivda?
Fotivda may cause serious side effects, including:
- High blood pressure (hypertension). High blood pressure is common with Fotivda and may sometimes be severe. Fotivda may also cause a sudden, severe increase in your blood pressure (hypertensive crisis) that can lead to death. Your healthcare provider should check your blood pressure after 2 weeks and at least monthly during treatment with Fotivda. Your healthcare provider may prescribe medicine to treat your high blood pressure if you develop blood pressure problems. You should check your blood pressure regularly during treatment with Fotivda and tell your healthcare provider if you have increased blood pressure. Tell your healthcare provider right away if you get any of the following signs or symptoms:
- confusion
- headaches
- dizziness
- chest pain
- shortness of breath
- Heart failure. Fotivda can cause heart failure which can be serious and sometimes lead to death. Your healthcare provider should check you for symptoms of heart failure regularly during treatment with Fotivda. Call your healthcare provider right away if you get symptoms of heart problems, such as shortness of breath or swelling of your ankles.
- Heart attack and blood clots in your veins or arteries. Fotivda can cause blood clots which can be serious and sometimes lead to death. Tell your healthcare provider or get emergency medical help right away if you get any of the following symptoms:
- new chest pain or pressure
- numbness or weakness on one side of your body
- pain in your arms, back, neck, or jaw
- trouble talking
- shortness of breath
- sudden severe headache
- vision changes
- swelling in the arms or legs
- Bleeding problems. Fotivda can cause bleeding which can be serious and sometimes lead to death. Tell your healthcare provider or get medical help right away if you develop any of the following signs or symptoms:
- unusual bleeding from the gums
- red or black stools (looks like tar)
- menstrual bleeding or vaginal bleeding that is heavier than normal
- bruises that happen without a known cause or get larger
- headaches, feeling dizzy or weak
- bleeding that is severe or you cannot control
- coughing up blood or blood clots
- pink or brown urine
- vomiting blood or your vomit looks like “coffee grounds”
- unexpected pain, swelling, or joint pain
- Protein in your urine. Your healthcare provider should check your urine for protein before and during your treatment with Fotivda.
- Thyroid gland problems. Your healthcare provider should do blood tests to check your thyroid gland function before and during your treatment with Fotivda. Your healthcare provider may prescribe medicine if you develop thyroid gland problems.
- Risk of wound healing problems. Wounds may not heal properly during Fotivda treatment. Tell your healthcare provider if you plan to have surgery before starting or during treatment with Fotivda, including dental surgery.
- You should stop taking Fotivda at least 24 days before planned surgery.
- Your healthcare provider should tell you when you may start taking Fotivda again after surgery.
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS). A condition called reversible posterior leukoencephalopathy syndrome (RPLS) can happen during treatment with Fotivda. Tell your healthcare provider right away if you get:
- headaches
- seizures
- confusion
- blindness or change in vision
- difficulty thinking
- Allergic reactions to tartrazine (FD&C Yellow No.5). Fotivda 0.89 mg capsules contain a dye called FD&C Yellow No.5 (tartrazine) which may cause allergic-type reactions, including bronchial asthma, in certain people. This allergic reaction is most often seen in people who also have an allergy to aspirin.
The most common side effects of Fotivda include:
- tiredness
- diarrhea
- decreased appetite
- nausea
- hoarseness
- low levels of thyroid hormones
- cough
- mouth sores
- decreased levels of salt (sodium) and phosphate in the blood
- increased levels of lipase in the blood (a blood test done to check your pancreas)
Other side effects include vomiting and weakness or lack of energy.
Fotivda may cause fertility problems in males and females, which may affect your ability to have a child. Talk to your healthcare provider if this is a concern for you.
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Fotivda if you have certain side effects.
These are not all of the possible side effects of Fotivda.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Fotivda
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Fotivda for a condition for which it was not prescribed. Do not give Fotivda to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Fotivda that is written for health professionals.
How should I store Fotivda?
- Store Fotivda at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Fotivda and all medicines out of the reach of children.
What are the ingredients in Fotivda?
Active ingredient: tivozanib hydrochloride
Inactive ingredients: mannitol and magnesium stearate.
The capsule contains gelatin, titanium dioxide, FDA yellow iron oxide, and Blue SB-6018 (ink). The 0.89 mg capsule also contains FD&C Blue #2 and Yellow SB-3017 (ink). The Yellow SB-3017 ink contains FD&C Yellow No.5 (tartrazine).
Label
PRINCIPAL DISPLAY PANEL – 0.89 MG CAPSULE BOTTLE LABEL
- NDC 45629-089-01
- FOTIVDA®
(tivozanib) capsules - 0.89 mg
- Contains color additives including
FD&C Yellow No. 5 (tartrazine) - AVEO
ONCOLOGY

PRINCIPAL DISPLAY PANEL – 1.34 MG CAPSULE BOTTLE LABEL

