Fleqsuvy
Generic name: baclofen
Dosage form: oral suspension
Medically reviewed by A Ras MD. Updated March 28, 2022
What is Fleqsuvy
Fleqsuvy (baclofen oral suspension) is a gamma-aminobutyric acid (GABA-ergic) agonist available as 25 mg per 5 mL (5 mg/mL) suspension for oral administration. Its chemical name is 4-amino-3-(4-chlorophenyl)-butanoic acid and its structural formula is: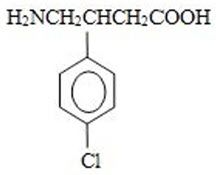
Molecular formula is C10H12C1NO2.
Molecular Weight is 213.66 g/mol.
Baclofen USP is a white to off-white, odorless, or practically odorless crystalline powder. It is slightly soluble in water, very slightly soluble in methanol, and insoluble in chloroform.
The Fleqsuvy (baclofen oral suspension) inactive ingredients are artificial grape flavor, citric acid anhydrous, D&C yellow No. 10, FD&C red No. 40, hydroxyethyl cellulose, propylene glycol, purified water, simethicone emulsion, sodium benzoate, and sucralose.¶
Mechanism of Action
The exact mechanism behind the baclofen’s action isn’t completely known. Baclofen blocks monosynaptic as well as polysynaptic responses in the spinal region, perhaps by reducing excitatory neurotransmitter release by Afferent terminals. However, actions at supraspinal locations could also be involved and contribute to its therapeutic effects. Baclofen is an analogous structure of the neurotransmitter inhibitory gamma-aminobutyric acid (GABA) and can be able to exert its effects via activation of GABAB receptor type.
Indications and utilization for Fleqsuvy
Fleqsuvy can be used for treating spasticity that is caused by multiple sclerosis, specifically to ease spasms of the flexor and discomfort, clonus, as well as muscle rigidity.
Fleqsuvy could also have some benefits for patients suffering from spinal cord injuries or other spinal cord disorders.
Limitations on Utilization
Fleqsuvy is not recommended for the treatment of muscle spasms caused by rheumatic diseases.
Doage and administration
Important Information
Fleqsuvy is an extremely concentrated formulation. Check the strength and dosage before prescribing, dispensing, or administering.
Recommended Dosage
Start Fleqsuvy by introducing a lower dose that is best in doses divided and taken orally. The following gradual increase in dosage regimen is recommended, but it should be adjusted depending on the response of patients and tolerability
1mL (5 mg) 3 times per every day for three days
2 milliliters (10 mg) 3 times per every day for three days
3mL (15 mg) three times per every day for three days
4.mL (20 mg) 3 times per every day for three days
It is possible to increase the dosage until the maximum recommended dose of 80 mg per day 4 mg (20 mg) every day, four times per day.
Administration Instructions
Shake thoroughly Fleqsuvy oral suspension prior to administering. Recycle any remaining portion after 2 months from the first opening.
A measuring device that is calibrated is recommended to determine and administer the prescribed dose precisely. A tablespoon or teaspoon from your kitchen is not a reliable measuring instrument.
Discontinuation of Fleqsuvy
If you are discontinuing Fleqsuvy reduce the dosage gradually and avoid abrupt withdrawal from the drug in order to lower the risk of negative reactions
Dosage Form and strength
Inhalation Suspension 25 mg / 5 milliliters (5 mg/mL) baclofen in an intense yellow to orange color, sweet-tasting grapes suspension.
Contraindication
Fleqsuvy is not recommended for patients who are hypersensitive to baclofen.
Warnings and precautions
Negative Reactions resulting from the abrupt Retraction of Fleqsuvy
The abrupt withdrawal of baclofen regardless of reason can cause negative reactions such as seizures, hallucinations, and hallucinations. They also have hyper-ever and altered mental state, hyper-intense rebound spasticity, and rigidity of the muscles, which in some cases, has led to rhabdomyolysis and multiple organ system failures, and even death. Therefore, lower the dosage gradually after Fleqsuvy is stopped unless the medical situation requires a fast withdrawal.
Neonatal withdrawal symptoms
In neonates, withdrawal symptoms who had mothers treated with baclofen in the course of pregnancy have been observed from hours to days after the delivery. The signs of withdrawal among these infants have included an increase in muscles, tremors as well as jitteriness, and seizures. If the benefits of the treatment outweigh the risks to the fetus and Fleqsuvy is used throughout pregnancy, slowly reduce the dose and cease Fleqsuvy prior to the birth. If a gradual withdrawal isn’t possible, notify the caregivers or parents of the neonate who is exposed to the potential for withdrawal of the neonate.
Sedation and Drowsiness
Sedation and drowsiness have been observed in as high as 60% of those who take baclofen which is the main ingredient of Fleqsuvy. Patients should stay clear of motor vehicles, or other hazardous machinery as well as activities that are a risk due to reduced alertness after taking Fleqsuvy, or increasing their dose until they are aware of how the drug affects them.
Be aware to be aware that central nervous system depressant effects of Fleqsuvy could be in addition to the effects of alcohol and other CNS depressants.
Insufficient Tolerability for Stroke Patients
Fleqsuvy must be taken with caution by patients who have suffered a stroke. Baclofen is not a significant benefit to stroke sufferers. Patients with stroke have also demonstrated low tolerance to the drug.
Exacerbation of Schizophrenia, Psychotic Disorders, or Confusional State
Fleqsuvy is recommended to be used with caution when treating those suffering from schizophrenia, psychotic disorders, or states of confusion. In the event of treatment with Fleqsuvy, such patients must be monitored closely since exacerbations of these disorders were observed following the administration of baclofen via the oral route.
Exacerbation of Autonomic Dysreflexia
Fleqsuvy is to be used cautiously in patients with an autonomic dysreflexia history. The presence of stimuli that cause nociceptive or sudden removal of Fleqsuvy can trigger an autonomic dysreflexia episode.
The exacerbation Epilepsy
Fleqsuvy must be administered with caution for patients suffering from epilepsy. A decrease in seizure control has been documented in patients who take baclofen.
The effects of posture and balance
Fleqsuvy must be utilized with caution when patients are suffering from spasticity is used to maintain an upright posture and maintain balance during the course of movement, or when spasticity is employed to gain more function.
Ovarian Cysts
An increase in the frequency of ovarian cysts has been observed in female rats who were treated with oral baclofen. Ovarian cysts have been identified through palpation in around 4 percent of the MS patients treated with oral baclofen for up to a year. Most of the time the cysts disappear on their own as patients continued to receive the medication. Ovarian cysts can be present in about 10% up to 5 percent of a typical female.
Fleqsuvy Side effects
A drug may have some undesired side effects in addition to its intended effects. Although not all of these side effects are likely to occur, if they do, medical treatment may be required.
If any of the following side effects develop, contact your doctor right away:
Less common or rare
- Bloody or dark urine
- chest pain
- clumsiness, unsteadiness, trembling, or other problems with muscle control
- fainting
- false sense of well-being
- mental depression or other mood changes
- pounding heartbeat
- ringing or buzzing in the ears
- seeing or hearing things that are not there
- skin rash or itching
- slurred speech or other speech problems
- swelling of the ankles
- unexplained muscle stiffness
- unusual excitement
Get emergency help immediately if any of the following symptoms of overdose occur:
Symptoms of overdose
- Blurred vision
- dizziness
- drowsiness
- irregular, fast or slow, or shallow breathing
- lightheadedness
- loss of strength or energy
- muscle pain or weakness
- pale or blue lips, fingernails, or skin
- seizures
- sleepiness or unusual drowsiness
- trouble breathing
- unusual weak feeling
There may be certain adverse effects that may not require medical treatment. As your body responds to the drug, these side effects may fade away during treatment. In addition, your health care provider may be able to advise you on how to avoid or mitigate some of these adverse effects. If any of the following side effects persist or become troublesome, or if you have any questions about them, consult your doctor:
More common
- Confusion
- constipation
- dizziness, faintness, or lightheadedness when getting up suddenly from a lying or sitting position
- headache
- increased need to urinate
- nausea
- passing urine more often
- sweating
- trouble sleeping
- unusual tiredness or weakness
Less common or rare
- Diarrhea
- loss of appetite
- muscle or joint pain
- numbness or tingling in the hands or feet
- stomach pain or discomfort
- stuffy nose
- weight gain
Other, not listed, adverse effects may occur in certain patients. Check with your healthcare provider if you detect any other side effects.
For medical advice on side effects, contact your doctor. You can contact the FDA at 1-800-FDA-1088 to report side effects.
Experiences in Clinical Trials
Since the clinical trials take place in diverse conditions, the rates of adverse reactions found in the clinical trials for a drug are not directly comparable to rates observed in trials conducted for another drug. They may not accurately reflect the actual rates in actual use.
The most frequent adverse reaction is temporary Drowsiness. In a controlled study of 175 participants, transient sleepiness was reported in 63% of the patients taking baclofen in comparison to 36% who were in the control group. Other adverse reactions that are common (up to 15 percent) include dizziness and weakness. Reactions that cause adverse reactions with more than 1% frequency are described in Table 1.
| ADVERSE REACTION | Percentage |
| Drool | 10-63% |
| Dizziness | 5-15% |
| Weakness | 5-15% |
| Nausea | 4-12% |
| Confusion | 1-11% |
| Hypotension | 0-9% |
| Headache | 4-8% |
| Insomnia | 2-7% |
| Constipation | 2-6% |
| Urinary Frequency | 2-6% |
| Fatigue | 2-4% |
Some adverse reactions, not included in Table 1 classified by a system of the body were also reported
Neuropsychiatric symptoms include euphoria, enthusiasm depression, hallucinations muscles pain, paresthesia as well as tinnitus, slurred speaking, coordination disorders stiffness, dystonia ataxia, blurred sight, strabismus, nystagmus diplopia, mydriasis dysarthria, epileptic seizures
Cardiovascular: dyspnea, palpitation, chest pain, syncope
Gastrointestinal: dry mouth anorexia, a taste disorder nausea, abdominal pain as well as diarrhea. a positive test for blood occult in the stool
Genitourinary: enuresis and dysuria, urinary retention, inability to ejaculate, impotence and nocturia, hematuria, and enuresis.
Other symptoms: rash, psoriasis, and ankle edema. Excessive weight gain, perspiration nasal congestion
The following tests in the laboratory have been identified as abnormal for patients taking baclofen increased SGOT as well as elevated alkaline-phosphatase and an increase in blood sugar.
Drugs and drugs interactions
CNS Depressants and Alcohol
Fleqsuvy may cause depression of the CNS as well as sedation and drowsiness which can be a result of the drug when combined with other CNS depressants, or alcohol.
Use specific population
Pregnancy
- Risk Summary
There isn’t enough data on the risks of major complications during pregnancy, birth defects or miscarriages, or any other material adverse events that are associated with the use Fleqsuvy among pregnant women. There are negative consequences for fetal outcomes that are associated with the withdrawal of baclofen post-delivery. Baclofen administered orally to pregnant rats led to an increase in the incidence of fetal structural anomalies at a dose also linked to maternal toxicities. The risk of background birth defects, as well as miscarriage for the specific population, is unknown. General, in the U.S. general population, the risk of background major birth defects and miscarriage in pregnancy that is clinically identified is 24% and 15-20 percent, respectively.
- Clinical Aspects
Neonatal and Fetal adverse reactions
Fleqsuvy could cause an increase in the likelihood of developing late-onset withdrawal symptoms from neonatal birth
- Animal Data
Baclofen administered orally has been found to increase the frequency of Omphaloceles (ventral hernias) in fetuses from rats that were given 13 times mg/kg, which is 3 times on a basis of mg/m2 the highest dose that is recommended for human use as well as a decrease in the intake of food as well as weight loss in dams. This condition was not evident in rabbits or mice.
Lactation
- Risk Summary
When doses are prescribed baclofen is found in the milk of humans. There are no studies in humans regarding the effects of baclofen in relation to milk production. In the case of withdrawal symptoms, they can be observed in infants who are breastfed when the maternal administration of Fleqsuvy ceases or breastfeeding ceases. There is no evidence of the effects of baclofen on a breastfeeding infant.
The benefits for the health and development of breastfeeding must be taken into consideration together with the mother’s clinical need for Fleqsuvy as well as any adverse consequences that might affect the baby who is breastfed due to Fleqsuvy or the conditions that cause the mother’s condition.
Use for Children
The safety and efficacy of children who are younger than 12 years of age are not yet established.
Geriatric Use
In general, dosage selection for patients with a chronic illness is a matter of caution, generally beginning at the lowest limit of the dosage range. This is due to the higher incidence of lower renal, hepatic, or cardiac activity, as well as of other concomitant diseases or medication therapy. The drug is known to be extensively excreted by the kidney. The possibility of adverse reactions to this medication is higher for patients who have impaired kidney function. Since elderly sufferers are more likely to suffer from diminished kidney function, care must be taken when deciding on doses and it is important to check renal function [see Use for Particular Groups
Renal Impairment
Since baclofen is mostly excreted unchanged by the kidneys Fleqsuvy must be administered cautiously to patients suffering from kidney impairment. It could be necessary to lower the dose.
Signs of Baclofen Overdose
In the event of an overdose of baclofen, patients could be in a coma or develop dizziness, lightheadedness, drowsiness somnolence, accommodation disorders, and seizures. They may also experience respiratory depression or hypotonia progressing to loss of consciousness.
Treatment for an Overdose
The treatment for baclofen overdose is to decontaminate the gastric area and maintain healthy breathing and airway.
Carcinogenesis Mutagenesis or Impairment of Fertility
Carcinogenesis
A decrease in tumors was not observed in rats who received baclofen in the form of a pill for 2 years at 30-60 times per mg/kg or between 10 and 20 using an mg/m2 scale the highest dose that is recommended for human use.
Mutagenesis
Genetic toxicology tests have not been done for baclofen.
Impairment of fertility
The effect of baclofen’s baclofen-based hormone on fertility has not been conducted.
How supplied/storage
how supplied
Fleqsuvy (baclofen oral suspension) has 25 mg of 5 milliliters (5 mg/mL) baclofen. This is an extremely concentrated orange-to yellow-colored grape-flavored suspension that is available in high-density polyethylene (HDPE) bottles that are white, polypropylene, and child-resistant closures that have an inner seal made of foam and a heat-induction-layered seal inside.
120 milliliters NDC 52652-6001-1
300 milliliters, NDC 52652-6001-2
Storing and handling
Keep at 20degC- 25degC (68degF to 77degF) The permitted range of excursions is 15deg to 30deg (59deg and 86deg F) (see USP Controlled Room Temperature).
Throw away any portion that is not used 2 months following the first opening.
Patient counseling
Administration Instructions
Instruct patients about Fleqsuvy, which is a concentrated formula. Inform caregivers or patients to use an oral dosage syringe instead of an ordinary teaspoon to accurately calculate the amount of medication. Instruct patients about the fact that oral dose Syringes can be purchased at their local pharmacy. Make sure patients shake before applying to dose.
Risks Associated with Sudden Withdrawal of Fleqsuvy
Inform patients and caregivers to stop taking Fleqsuvy before consulting their physician because abrupt discontinuation of Fleqsuvy could cause serious problems that can include hallucinations and seizures as well as high fever, muscular stiffness, confusion multi-organ system failure, and even death. Inform patients that the initial signs of Fleqsuvy withdrawal can include an increase in spasticity, itching, and tingling in the extremities.
Signs and symptoms of withdrawal from the Neonatal Unit
Encourage patients to inform their physician when they are pregnant or are planning to be pregnant, or are planning to start breastfeeding.
A higher risk of Drowsiness Alcohol and other CNS depressants
Be aware that Fleqsuvy could cause drowsiness. Also, they should be cautious about the use of motor vehicles, other potentially dangerous equipment, or any activities that are a risk due to decreased alertness after starting FLEQSUVY, or increasing dosage until they are aware of what the effects of the drug are. Inform caregivers and patients that the drowsiness resulting from Fleqsuvy can be exacerbated by alcohol or other depressants of the CNS. Encourage patients to read all prescription labels thoroughly and inform their physician about all medications, both prescription and non-prescription, they take.
Label
- NDC 52652-60001-1
- Rx Only
- FLEQSUVY™ (baclofen oral suspension)
- 25 mg per 5 mL (5 mg/mL) Concentrated Formulation

