Empaveli
Generic name: pegcetacoplan
Dosage form: injection, for subcutaneous use
Drug class: Selective immunosuppressants
Medically reviewed by A Ras MD.
What is Empaveli?
Empaveli is a prescription medicine used to treat adults with a disease called paroxysmal nocturnal hemoglobinuria (PNH). It is not known if Empaveli is safe and effective in children.
Description
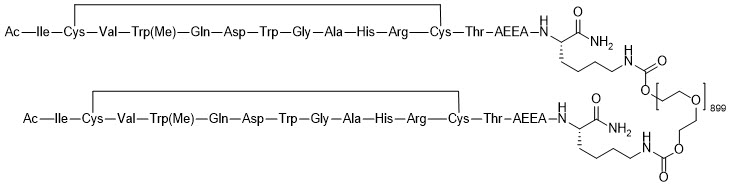
EMPAVELI contains pegcetacoplan, a complement inhibitor. Pegcetacoplan is a symmetrical molecule comprised of two identical pentadecapeptides covalently bound to the ends of a linear 40-kiloDalton (kDa) PEG molecule. The peptide portions of pegcetacoplan contain 1-methyl-L-tryptophan (Trp(Me)) in position 4 and amino(ethoxyethoxy)acetic acid (AEEA) in position 14.
The molecular weight of pegcetacoplan is approximately 43.5 kDa. The molecular formula is C1970H3848N50O947S4. The structure of pegcetacoplan is shown below.
EMPAVELI injection is a sterile, clear, colorless to slightly yellowish aqueous solution for subcutaneous use and is supplied in a 20-mL single-dose vial. Each 1 mL of solution contains 54 mg of pegcetacoplan, 41 mg of sorbitol, 0.384 mg of glacial acetic acid, 0.490 mg of sodium acetate trihydrate, and Water for Injection USP. EMPAVELI may also contain sodium hydroxide and/or additional glacial acetic acid for adjustment to a target pH of 5.0.
Mechanism of Action
Pegcetacoplan binds to complement protein C3 and its activation fragment C3b, thereby regulating the cleavage of C3 and the generation of downstream effectors of complement activation. In PNH, extravascular hemolysis (EVH) is facilitated by C3b opsonization while intravascular hemolysis (IVH) is mediated by the downstream membrane attack complex (MAC). Pegcetacoplan acts proximally in the complement cascade controlling both C3b-mediated EVH and terminal complement-mediated IVH.
What is the most important information I should know about Empaveli?
Empaveli is a medicine that can affect your immune system. Empaveli can lower the ability of your immune system to fight infections.
- Empaveli may increase your chance of getting serious and life-threatening meningococcal infections. Meningococcal infections may quickly become life-threatening and cause death if not recognized and treated early.
- Empaveli may also increase the risk of getting serious infections. People who take Empaveli may have an increased risk of getting infections caused by Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B. Serious infections may quickly become life-threatening and cause death if not recognized and treated early.
- You must be vaccinated against these bacteria at least 2 weeks before your first dose of Empaveli if you have not already had these vaccines.
- If your healthcare provider decides that urgent treatment with Empaveli is needed, you should receive the required vaccinations as soon as possible.
- If you have not been vaccinated and Empaveli therapy must be initiated immediately, you should also receive 2 weeks of antibiotics with your vaccinations.
- If you have been vaccinated against these bacteria in the past, you might need additional vaccinations before starting Empaveli. Your healthcare provider will decide if you need additional vaccinations.
- Vaccines reduce the risk of serious infections, but do not prevent all serious infections. Call your healthcare provider or get emergency medical care right away if you get any of these signs and symptoms of a serious infection:
- fever with or without shivers or the chills
- fever and a rash
- shortness of breath
- extreme pain or discomfort
- headache with nausea or vomiting
- high heart rate
- headache and a fever
- headache with a stiff neck or stiff back
- confusion
- muscle aches with flu-like symptoms
- clammy skin
- eyes sensitive to light
Your healthcare provider will give you a Patient Safety Card about the risk of serious infections. Carry it with you at all times during treatment and for 2 months after your last Empaveli dose. Your risk of serious infections may continue for several weeks after your last dose of Empaveli. It is important to show this card to any healthcare provider who treats you. This will help them diagnose and treat you quickly.
Empaveli is only available through a program called the Empaveli REMS. Before you can take Empaveli, your healthcare provider must:
- enroll in the Empaveli REMS program.
- counsel you about the risk of serious infections caused by certain bacteria.
- give you information about the symptoms of serious infections.
- give you a Patient Safety Card about your risk of serious infections, as discussed above.
- make sure that you are vaccinated.
Who should not use Empaveli?
Do not take Empaveli if you:
- are allergic to pegcetacoplan or any of the ingredients in Empaveli. See the end of this Medication Guide for a complete list of ingredients in Empaveli.
- have not been vaccinated against Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B, unless your healthcare provider decides that urgent treatment with Empaveli is needed. See “What is the most important information I should know about Empaveli?”
- have a serious Streptococcus pneumoniae, Neisseria meningitidis, or Haemophilus influenzae type B infection.
What should I tell my healthcare provider before using Empaveli?
Before you take Empaveli, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection or fever.
- are pregnant or plan to become pregnant. Empaveli may harm your unborn baby. Females who are able to become pregnant should have a pregnancy test before starting treatment with Empaveli.
- Females who are able to become pregnant should use an effective method of birth control (contraception) during treatment with Empaveli and for 40 days after the final dose.
- are breastfeeding or plan to breastfeed. It is not known if Empaveli passes into your breast milk. You should not breastfeed during treatment with Empaveli and for 40 days after the final dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Empaveli and other medicines can affect each other, causing side effects.
Know the medicines you take and the vaccines you receive. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Empaveli?
- See the detailed Instructions for Use that comes with your Empaveli for information about how to prepare and infuse your dose of Empaveli.
- Your healthcare provider should show you how to prepare and infuse Empaveli before you use it for the first time.
- Use Empaveli exactly as your healthcare provider tells you. Your healthcare provider will tell you how much Empaveli to infuse and how often to infuse Empaveli. Do not infuse more or less than your healthcare provider tells you to.
- Empaveli is given by infusion under the skin (subcutaneously) into your stomach (abdomen), back of upper arms, hips, or thighs using an infusion pump.
- Empaveli is given by an infusion 2 times each week. If there is an increase in your LDH, an enzyme in your blood, your healthcare provider may tell you to take Empaveli every 3 days.
- If you are changing treatment from eculizumab to Empaveli, you should continue eculizumab for 4 weeks after your first dose of Empaveli. After 4 weeks, you should stop treatment with eculizumab.
- If you are changing treatment from ravulizumab to Empaveli, you should take your starting dose of Empaveli no more than 4 weeks after your last dose of ravulizumab.
- If you have PNH and you stop taking Empaveli, your healthcare provider will need to monitor you closely for at least 8 weeks after stopping Empaveli. Stopping treatment with Empaveli may cause a breakdown of red blood cells due to PNH.
Symptoms or problems that can happen due to red blood cell breakdown include:- decreased hemoglobin level in your blood
- blood in your urine
- shortness of breath
- trouble swallowing
- tiredness
- pain in the stomach (abdomen)
- blood clots
- erectile dysfunction (ED)
If you miss a dose of Empaveli, take the missed dose as soon as possible. Take your next dose at your regularly scheduled time.
What are the possible side effects of Empaveli?
Empaveli can cause serious side effects including:
- See “What is the most important information I should know about Empaveli?”
- Allergic reactions. Allergic reactions can happen during your Empaveli infusion. Stop your Empaveli infusion and tell your healthcare provider or get emergency medical care right away if you get any of these symptoms during your Empaveli infusion:
- chest pain
- trouble breathing or shortness of breath
- swelling of your face, tongue, or throat
- feel faint or pass out
The most common side effects in people with PNH treated with Empaveli include injection-site reactions, infections, diarrhea, pain in the stomach (abdomen), respiratory tract infection, viral infection, and tiredness.
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all of the possible side effects of Empaveli. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Empaveli
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Empaveli for a condition for which it was not prescribed. Do not give Empaveli to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Empaveli that is written for health professionals.
How should I store Empaveli?
- Store vials of Empaveli in the refrigerator between 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
- Do not use Empaveli past the expiration date stamped on the carton.
Keep Empaveli and all medicines out of the reach of children.
What are the ingredients in Empaveli?
Active ingredient: pegcetacoplan
Inactive ingredients: sorbitol, glacial acetic acid, sodium acetate trihydrate, Water for Injection USP. Empaveli may also contain sodium hydroxide and/or additional glacial acetic acid for pH adjustment.
For more information, go to www.EMPAVELI.com or call 1-866-692-7527
Instructions for use for Empaveli
Empaveli (em-puh-vel-ee)
(pegcetacoplan)
Injection, for subcutaneous use
Important Information
Read this Instructions for Use before you start using Empaveli and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. Your healthcare provider should show you or your caregiver how to infuse Empaveli the right way before you use it for the first time. Ask your healthcare provider about any instructions you do not understand.
How should I store Empaveli?
- Store vials of Empaveli in the refrigerator between 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
- Do not use Empaveli past the expiration date stamped on the carton.
Keep Empaveli and all medicines out of the reach of children.
| Step 1 | Prepare for infusion Before you start:
|
|
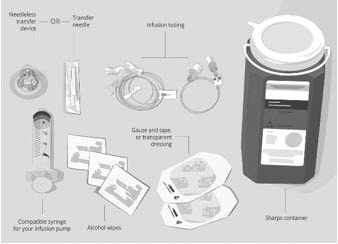
Gather your supplies (See Figure A):
|
Figure A: Supplies
|
|
| Clean your work surface well using an alcohol wipe. | ||
| Wash your hands well with soap and water. Dry your hands. | ||
| Step 2 | Check the vial and liquid
Do not use the vial if:
|
Figure B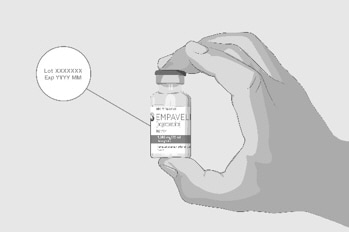 |
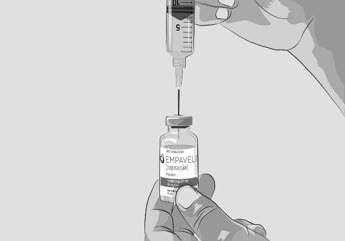
| Step 3 | Prepare and fill syringe
|
Figure C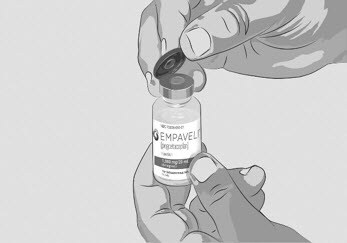 |
| Option 1: If using a needleless transfer device (such as a vial adapter), follow the instructions provided by the device manufacturer. OR Option 2: If transfer is done using a transfer needle and a syringe, follow the instructions below:
|
Figure D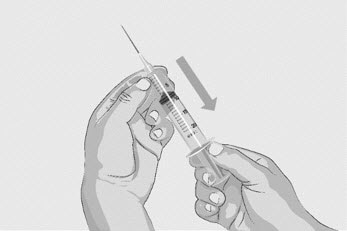
Figure E |
|
|
Figure F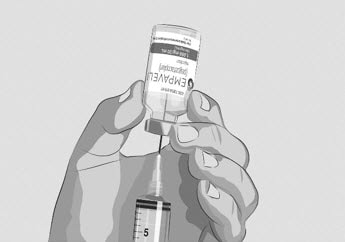 |
|
|
Figure G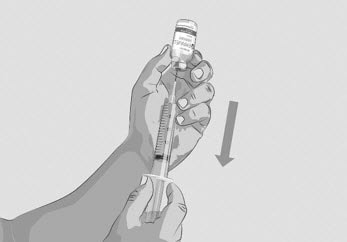 |
|
|
Figure H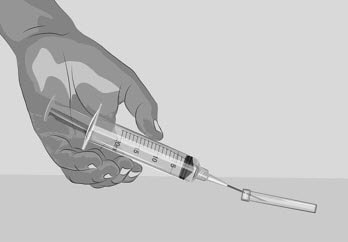 |
|
|
Figure I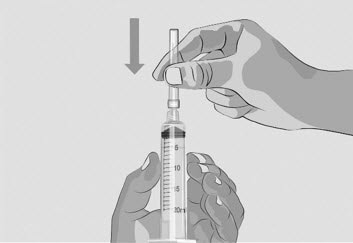 |
|
|
Figure J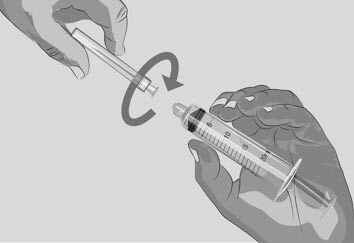 |
|
| Step 4 | Prepare infusion pump and tubing
|
|
| Step 5 | Prepare the infusion site(s)
|
Figure K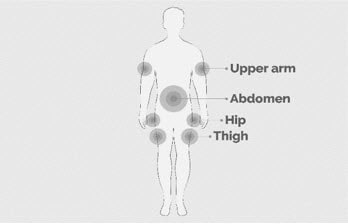 |
|
Figure L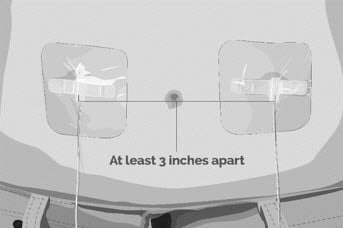 |
|
|
Figure M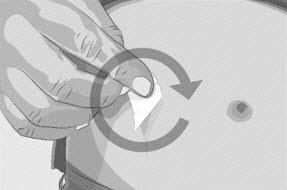 |
|
| Step 6 | Insert and secure the infusion needle(s)
|
Figure N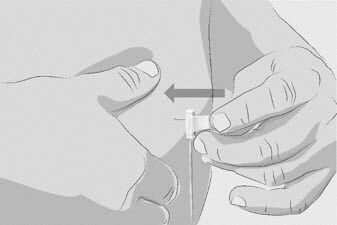 |
|
Figure O
|
|
| Step 7 | Start infusion
|
|
| Step 8 | Complete infusion
|
|
| Step 9 | Record infusion
|
|
| Step 10 | Clean up
|
|
| Step 11 | Dispose of (throw away) used needles and syringes and Empaveli infusion tubing.
|
Label
PRINCIPAL DISPLAY PANEL – 1,080 MG/20 ML VIAL CARTON
- NDC 73606-010-01
- EMPAVELI™
(pegcetacoplan)
Injection - 1,080 mg/20 mL
(54 mg/mL) - For Subcutaneous Infusion Only
- Dispense the enclosed
Medication Guide to each patient. - One 20 mL Single-Dose Vial.
Discard unused portion. - Rx only
- Apellis

SRC: NLM .