Daytrana
Generic name: methylphenidate (transdermal)
Brand name: Daytrana
Drug class: CNS stimulants
Medically reviewed by A Ras MD.
What is Daytrana?
Daytrana is a prescription medicine used to treat Attention Deficit Hyperactivity Disorder (ADHD) in people 6 to 17 years old. Daytrana is a central nervous system (brain) stimulant medicine. Daytrana may help you have better attention and less impulsive and hyperactive behavior. Daytrana is a patch that you apply to your skin on your hip. Daytrana is used as part of a total treatment program for ADHD that may also include counseling or other treatments.
It is not known if Daytrana is safe and effective in children younger than 6 years.
Description
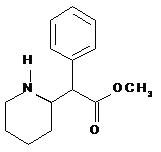
DAYTRANA is an adhesive-based matrix transdermal system (patch) containing methylphenidate that is applied to intact skin. The chemical name for methylphenidate is α-phenyl-2-piperidineacetic acid methyl ester. It is a white to off-white powder and is soluble in alcohol, ethyl acetate, and ether. Methylphenidate is practically insoluble in water and petrol ether. Its molecular weight is 233.31. Its empirical formula is C14H19NO2. The structural formula of methylphenidate is:
11.1 Patch Components
DAYTRANA contains methylphenidate in a multipolymeric adhesive. The methylphenidate is dispersed in acrylic adhesive that is dispersed in a silicone adhesive. The composition per unit area of all dosage strengths is identical, and the total dose delivered is dependent on the patch size and wear time.
The patch consists of three layers, as seen in the figure below (cross-section of the patch).
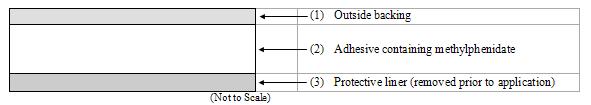 Proceeding from the outer surface toward the surface adhering to the skin, the layers are (1) a polyester/ethylene vinyl acetate laminate film backing, (2) a proprietary adhesive formulation incorporating Noven Pharmaceuticals, Inc.’s DOT Matrix™ transdermal technology consisting of an acrylic adhesive, a silicone adhesive, and methylphenidate, and (3) a fluoropolymer-coated polyester protective liner, which is attached to the adhesive surface and must be removed before the patch can be used.
Proceeding from the outer surface toward the surface adhering to the skin, the layers are (1) a polyester/ethylene vinyl acetate laminate film backing, (2) a proprietary adhesive formulation incorporating Noven Pharmaceuticals, Inc.’s DOT Matrix™ transdermal technology consisting of an acrylic adhesive, a silicone adhesive, and methylphenidate, and (3) a fluoropolymer-coated polyester protective liner, which is attached to the adhesive surface and must be removed before the patch can be used.
The active component of the patch is methylphenidate. The remaining components are pharmacologically inactive.
Mechanism of Action
Methylphenidate is a central nervous system (CNS) stimulant. Its mode of therapeutic action in Attention Deficit Hyperactivity Disorder (ADHD) is not known.
What is the most important information I should know about Daytrana?
Only Use Daytrana on Your Skin
Daytrana is a federally controlled substance (CII) because it can be abused or lead to dependence. Keep Daytrana in a safe place to protect it from theft. Selling or giving away Daytrana may harm others and is against the law.
Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicine or street drugs.
Daytrana is a central nervous system (brain) stimulant medicine. Serious side effects have been reported with Daytrana or other stimulant medicines, including:
1. Heart problems, including:
- sudden death in people who have heart problems or heart defects
- stroke and heart attack in adults
- increased blood pressure and heart rate
Your doctor should check you carefully for blood pressure and heart problems before you start and while you are using Daytrana.
Remove the Daytrana patch and call your doctor right away if you have any signs of heart problems such as:
- chest pain
- shortness of breath
- fainting
2. Mental (psychiatric) problems, including:
- new or worse aggressive behavior, hostility, anger, or irritability
- new or worse bipolar illness or mania (an extreme increase in activity or talking)
- new or worse psychosis (hearing or seeing things that are not real, being suspicious or distrustful, believing things that are not true)
- other unusual or extreme changes in behavior or mood
Tell your doctor right away if you have any new or worsening mental problems while using Daytrana.
3. Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud’s phenomenon]: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red
- Tell your doctor if you have or your child has numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes.
- Call your doctor right away if you have or your child has any signs of unexplained wounds appearing on fingers or toes while taking Daytrana
Who should not use Daytrana?
Do not use Daytrana if you:
- are very anxious, tense, or agitated
- have glaucoma
- have tics (repeated movements or sounds that cannot be controlled)
- have Tourette’s Syndrome or a family history of this syndrome
- are taking or have taken a monoamine oxidase inhibitor (MAOI) medicine within the past 2 weeks. Do not take a MAOI medicine for at least 2 weeks before using Daytrana. Ask your doctor or pharmacist if you are not sure if any of your medicines are MAOIs.
- are allergic to methylphenidate or any other ingredients in Daytrana. See “What are the ingredients in Daytrana?” for a complete list of ingredients.
Talk to your healthcare provider before taking this medicine if you have any of these conditions.
What should I tell my healthcare provider before using Daytrana?
Before you start using Daytrana, tell your doctor if you:
- have heart problems, heart defects, high blood pressure
- have mental problems including psychosis, mania, bipolar illness, or depression
- have seizures or have had an abnormal brain wave test (EEG)
- have circulation problems in fingers or toes
- have skin problems such as eczema or psoriasis, or have skin reactions to soaps, lotions, make-up, or adhesives (glues)
- are pregnant or plan to become pregnant. It is not known if Daytrana will harm your unborn baby.
- There is a pregnancy registry for females who are exposed to ADHD medications, including Daytrana during pregnancy. The purpose of the registry is to collect information about the health of females exposed to Daytrana and their baby. If you or your child becomes pregnant during treatment with Daytrana, talk to your healthcare provider about registering with the National Pregnancy Registry of ADHD Medications at 1-866-961-2388 or visit online at https://womensmentalhealth.org/adhd-medications/.
- are breast feeding or plan to breast feed. Daytrana passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with Daytrana.
- a history of vitiligo and/or a family history of vitiligo
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Daytrana and certain other medicines may affect each other, causing serious side effects.
Especially tell your doctor if you take:
- a monoamine oxidase inhibitor (MAOI) medicine See “Who should not take Daytrana?”
- medicines to treat depression
- medicines to treat seizures
- a blood pressure medicine
- a blood thinner medicine
- cold or allergy medicines that contain decongestants
Know the medicines that you take. Keep a list of them to show your doctor and pharmacist. Do not start any new medicine while using Daytrana without talking to your doctor first.
How should I use Daytrana?
- Read the Patient Instructions for Use that comes with your Daytrana for information about the right way to use Daytrana.
- Use Daytrana exactly as your doctor tells you to.
- Your doctor may change your dose if needed.
- Apply Daytrana to your hip 2 hours before an effect is needed.
- Do not wear Daytrana longer than 9 hours a day.
- Apply Daytrana to a different hip each day.
- Do not cut Daytrana patches.
- Parents or caregivers should apply and remove Daytrana for their child if the child is not responsible enough to do so.
- Your doctor may stop Daytrana treatment to check your ADHD symptoms.
- Your doctor may do certain blood tests and check your heart and blood pressure while you use Daytrana.
- If you forget to apply a patch in the morning, you may apply the patch later in the day. You should remove your patch at the usual time of day to lower the chance of side effects later in the day.
- If you have loss of appetite or trouble sleeping in the evening, ask your doctor if you can take the patch off earlier in the day.
- Contact with water while bathing, swimming, or showering can make the patch not stick well or make it fall off. If your patch falls off, do not touch the sticky side of the patch with your fingers. You may apply a new patch to a different area on the same hip. If you have to replace a patch that has fallen off, the total wear time for the first and second patch should not be more than a total of 9 hours in 1 day. Do not reapply the same patch that fell off.
- Do not wear your Daytrana patch longer than 9 hours.
- If you accidentally apply or wear more than 1 patch at a time, you have used too much Daytrana. Remove all patches, wash the application sites right away and call your doctor.
- Call your Poison Control Center at 1-800-222-1222 -or go to the nearest hospital emergency room right away if you have:
- vomiting
- agitation
- shaking
- confusion or mental changes
- see things that are not there (hallucinations)
- sweating
- redness in your face
- headache
- heartbeat changes
- Call your Poison Control Center at 1-800-222-1222 -or go to the nearest hospital emergency room right away if you have:
What should I avoid while taking Daytrana?
- Do not put any medicine, cream, or lotion on your hip before you apply the Daytrana patch. Medicines, creams or lotions may affect how the patch sticks to your skin and how the medicine is absorbed from the patch.
- Do not use bandages, tape, or other household adhesives (glue) to hold the patch onto your skin.
- Do not use hair dryers, heating pads, electric blankets, heated water beds or other heat sources while wearing a Daytrana patch. Too much medicine can pass into your body and cause serious side effects.
- Do not drive, operate heavy machinery or do other dangerous activities until you know how Daytrana affects you.
What are the possible side effects of Daytrana?
Daytrana may cause serious side effects, including:
- See “What is the most important information I should know about Daytrana?”
- Seizures. This usually happens in people with a history of seizures.
- Painful and prolonged erections (priapism) have occurred with methylphenidate. If you or your child develops priapism, seek medical help right away. Because of the potential for lasting damage, priapism should be evaluated by a doctor immediately.
- Slowing of growth (weight and height). You should have your height and weight checked while using Daytrana.
- Loss of skin color (chemical leukoderma). Daytrana may cause a persistent loss of skin-color where the patch is applied or around the patch application site. Loss of skin-color, in some cases, has been reported at locations on the skin far from any application site. The loss of your skin-color may be permanent even after removing the patch or stopping use of Daytrana. Call your doctor immediately if you have changes in your skin-color.
- Allergic skin rash. Stop using Daytrana and see your doctor right away if you have swelling or blisters at or around the application site. You may have a skin allergy to Daytrana. People who have skin allergies to Daytrana may develop an allergy to all medicines that contain methylphenidate, even those methylphenidate medicines that are taken by mouth.
- Eyesight changes or blurred vision
The most common side effects of Daytrana include:
- skin problems where you apply Daytrana (redness, small bumps, itching)
- poor appetite
- nausea
- vomiting
- stomach pain
- weight loss
- tics
- trouble sleeping
- mood swings
- dizziness
Tell your doctor if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of Daytrana. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Daytrana
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Daytrana for a condition for which it was not prescribed. Do not give Daytrana to other people, even if they have the same symptoms that you have. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about Daytrana. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Daytrana that is written for health professionals.
For more information, go to www.daytrana.com, or call 1-877-567-7857.
How should I store Daytrana?
- Store Daytrana at room temperature between 68° F to 77° F (20° C to 25° C).
- Do not store Daytrana in the refrigerator or freezer.
- Keep Daytrana patches in their unopened pouches until you are ready to use them.
- Use or throw away the patches within 2 months after you open the sealed tray or outer pouch.
Keep Daytrana and all medicines out of the reach of children.
What are the ingredients in Daytrana?
Active ingredient: methylphenidate
Inactive ingredients: acrylic adhesive, silicone adhesive
Label
PRINCIPAL DISPLAY PANEL – NDC 68968-5552-3 – 10 mg 30 Count Carton

SRC: NLM .
