Daurismo
Generic name: glasdegib
Drug class: Hedgehog pathway inhibitors
Medically reviewed by A Ras MD.
What is Daurismo?
Daurismo is a prescription medicine that is used with the medicine cytarabine to treat newly-diagnosed acute myeloid leukemia (AML) in adults who are 75 years of age or older, or have other medical conditions that prevent the use of standard chemotherapy
It is not known if Daurismo is safe and effective in children.
Description
DAURISMO (glasdegib) is a potent small molecule inhibitor of Smoothened (SMO) for oral use. It is formulated with the maleate salt of glasdegib. The molecular formula for glasdegib maleate is C25H26N6O5. The molecular weight for glasdegib maleate is 490.51 Daltons. The chemical name of glasdegib maleate is 1-((2R,4R)-2-(1H-benzo[d]imidazol-2-yl)-1-methylpiperidin-4-yl)-3-(4-cyanophenyl)urea maleate. The molecular structure is shown below:
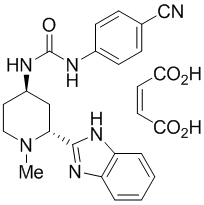
Glasdegib maleate is a white to pale colored powder with pKa values of 1.7 and 6.1. The aqueous solubility of glasdegib maleate is 1.7 mg/mL.
DAURISMO (glasdegib) is supplied as a film-coated tablet for oral use containing either 100 mg glasdegib (equivalent to 131.1 mg glasdegib maleate) or 25 mg of glasdegib (equivalent to 32.8 mg glasdegib maleate) together with microcrystalline cellulose, dibasic calcium phosphate anhydrous, sodium starch glycolate, and magnesium stearate as inactive ingredients in the tablet. The film-coating consists of Opadry II® Beige (33G170003) and Opadry II® Yellow (33G120011) containing: hypromellose, titanium dioxide, lactose monohydrate, macrogol, triacetin, iron oxide yellow, and iron oxide red.
Mechanism of Action
Glasdegib is an inhibitor of the Hedgehog pathway. Glasdegib binds to and inhibits Smoothened, a transmembrane protein involved in hedgehog signal transduction.
In a murine xenotransplant model of human AML, glasdegib in combination with low-dose cytarabine, inhibited increases in tumor size and reduced the percentage of CD45+/CD33+ blasts in the marrow to a greater extent than glasdegib or low-dose cytarabine alone.
What is the most important information I should know about Daurismo?
Daurismo can cause your baby to die before it is born (be stillborn) or cause your baby to have severe birth defects.
For females who can become pregnant:
- You should talk to your healthcare provider about the risks of Daurismo to your unborn child.
- Your healthcare provider will do a pregnancy test within 7 days before you start taking Daurismo.
- You should not use Daurismo during pregnancy.
- You should use effective birth control during treatment and for at least 30 days after your last dose of Daurismo. Talk with your healthcare provider about what birth control method is right for you during this time.
- Talk to your healthcare provider right away if you have unprotected sex or if you think that your birth control has failed.
- Tell your healthcare provider right away if you become pregnant or think that you may be pregnant.
For males:
- It is not known if Daurismo is present in semen. Do not donate semen during treatment with Daurismo and for at least 30 days after your last dose.
- You should always use effective birth control, including a condom, even if you have had a vasectomy, during sex with female partners who are pregnant or who are able to become pregnant, during treatment with Daurismo and for at least 30 days after your last dose to protect your female partner from being exposed to Daurismo.
- Tell your healthcare provider right away if your partner becomes pregnant or thinks she is pregnant while you are taking Daurismo.
Exposure to Daurismo during pregnancy:
If you think that you or your female partner may have been exposed to Daurismo during pregnancy, talk to your healthcare provider right away. If you become pregnant during treatment with Daurismo, you or your healthcare provider should report your pregnancy to Pfizer at 1-800-438-1985.
What should I tell my healthcare provider before taking Daurismo?
Before you take Daurismo, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems, including a condition called long QT syndrome.
- abnormal blood salt (electrolytes) levels.
- are pregnant or plan to become pregnant. See “What is the most important information I should know about Daurismo?”
- are breastfeeding or plan to breastfeed. It is not known if Daurismo passes into your breast milk. Do not breastfeed or provide breast milk to infants or children during treatment with Daurismo and for at least 30 days after the last dose. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements.
How should I take Daurismo?
- Take Daurismo with the medicine cytarabine exactly as your healthcare provider tells you.
- Take Daurismo 1 time each day, at about the same time each day.
- Take Daurismo with or without food.
- Do not chew, split or crush Daurismo tablets.
- If you miss a dose, take it as soon as you remember. If it is less than 12 hours before your next dose, just skip the missed dose and take your next dose at your regular time. Do not take 2 doses of Daurismo within 12 hours.
- If you vomit after taking a dose of Daurismo, do not take an extra dose, just take your next dose at your regular time.
- Your healthcare provider will perform certain tests to check you for side effects before and during treatment with Daurismo.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Daurismo if you have certain side effects. Do not change your dose or stop taking Daurismo unless your healthcare provider tells you.
What should I avoid while taking Daurismo?
- Do not donate blood or blood products during treatment with Daurismo and for at least 30 days after the last dose.
- Do not donate semen during treatment with Daurismo and for at least 30 days after the last dose.
What are the possible side effects of Daurismo?
Daurismo can cause serious side effects, including:
- See “What is the most important information I should know about Daurismo?”
- Changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heartbeats that can be life-threatening. Tell your healthcare provider right away if you feel faint, lightheaded, dizzy, or feel your heart beating irregularly or fast during treatment with Daurismo.
The most common side effects of Daurismo with cytarabine include:
- low red blood cell count (anemia)
- tiredness
- bleeding
- fever with low white blood cell count
- muscle pain
- nausea
- swelling of arms or legs
- low platelet count
- shortness of breath
- decreased appetite
- changes in taste
- pain or sores in your mouth or throat
- constipation
- rash
Daurismo may affect fertility in males. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of Daurismo.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Daurismo
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Daurismo for a condition for which it was not prescribed. Do not give Daurismo to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about Daurismo that is written for health professionals.
How should I store Daurismo?
- Store Daurismo at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Daurismo and all medicines out of the reach of children.
What are the ingredients in Daurismo?
Active ingredient: glasdegib
Inactive ingredients: microcrystalline cellulose, dibasic calcium phosphate anhydrous, sodium starch glycolate, and magnesium stearate.
Film-coating:
25 mg tablets: Opadry II Yellow (33G120011) containing hypromellose, titanium dioxide, lactose monohydrate, macrogol, triacetin, and iron oxide yellow.
100 mg tablets: Opadry II Beige (33G170003) containing hypromellose, titanium dioxide, lactose monohydrate, macrogol, triacetin, iron oxide yellow, and iron oxide red.
Label
PRINCIPAL DISPLAY PANEL – 25 MG TABLET BOTTLE LABEL
- ALWAYS DISPENSE
WITH MEDICATION GUIDE - NDC 0069-0298-60
- Pfizer
- Daurismo™
(glasdegib) tablets - 25 mg*
- Do not cut, crush, or chew the tablets.
- 60 Tablets
Rx only

PRINCIPAL DISPLAY PANEL – 100 MG TABLET BOTTLE LABEL
- ALWAYS DISPENSE
WITH MEDICATION GUIDE - NDC 0069-1531-30
- Pfizer
- Daurismo™
(glasdegib) tablets - 100 mg*
- Do not cut, crush, or chew the tablets.
- 30 Tablets
Rx only

SRC: NLM .
