Corlanor
eneric name: ivabradine
Drug class: Miscellaneous cardiovascular agents
Medically reviewed by A Ras MD.
What is Corlanor?
Corlanor is a prescription medicine used to treat adults who have chronic (lasting a long time) heart failure, with symptoms, to reduce their risk of hospitalization for worsening heart failure, to treat certain children 6 months of age and older who have stable heart failure, with symptoms, that is due to an enlarged heart (dilated cardiomyopathy).
Descriptioin
Corlanor (ivabradine) tablets and oral solution contains ivabradine as the active pharmaceutical ingredient. Ivabradine is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that reduces the spontaneous pacemaker activity of the cardiac sinus node by selectively inhibiting the If current, resulting in heart rate reduction with no effect on ventricular repolarization and no effects on myocardial contractility.
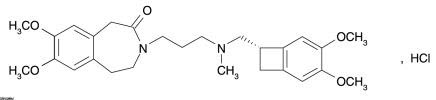
The chemical name for ivabradine hydrochloride is 3-(3-{[((7S)-3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methyl] methyl amino} propyl)-1,3,4,5-tetrahydro-7,8-dimethoxy-2H-3-benzazepin-2-one, hydrochloride. The molecular formula is C27H36N2O5∙HCl, and the molecular weight (free base + HCl) is 505.1 (468.6 + 36.5). The chemical structure of ivabradine is shown in Figure 1.
Figure 1. Chemical Structure of Ivabradine
Tablets
Corlanor tablets are supplied in 5 mg and 7.5 mg tablets for oral administration. The tablets contain 5 mg and 7.5 mg of ivabradine, as the active ingredient, equivalent to 5.39 mg and 8.09 mg of ivabradine hydrochloride, respectively. The tablets contain the following inactive ingredients: colloidal silicon dioxide, glycerol, hypromellose, lactose monohydrate, magnesium stearate, maize starch, maltodextrin, polyethylene glycol 6000, red iron oxide, titanium dioxide, and yellow iron oxide.
Oral Solution
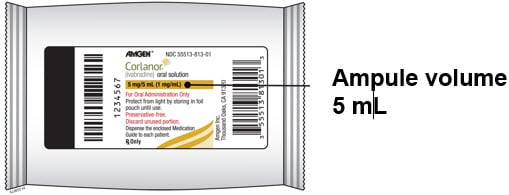
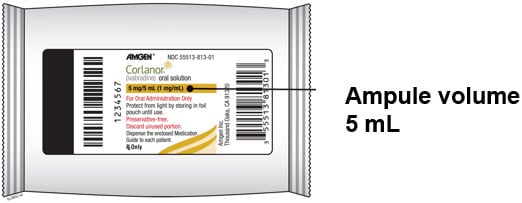
Corlanor 5 mg/5 mL (1 mg/mL) oral solution is formulated as a sterile, preservative-free, colorless solution for oral administration. Each 5 mL ampule contains 5 mg of ivabradine, equivalent to 5.39 mg ivabradine hydrochloride, as the active ingredient. The solution contains the following inactive ingredients: maltitol and water.
Mechanism of Action
Corlanor blocks the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel responsible for the cardiac pacemaker If current, which regulates heart rate. In clinical electrophysiology studies, the cardiac effects were most pronounced in the sinoatrial (SA) node, but prolongation of the AH interval has occurred as has PR interval prolongation. There was no effect on ventricular repolarization and no effects on myocardial contractility.
Corlanor can also inhibit the retinal current Ih. Ih is involved in curtailing retinal responses to bright light stimuli. Under triggering circumstances (e.g., rapid changes in luminosity), partial inhibition of Ih by Corlanor may underlie the luminous phenomena experienced by patients. Luminous phenomena (phosphenes) are described as a transient enhanced brightness in a limited area of the visual field
What is the most important information I should know about Corlanor?
Corlanor may cause serious side effects in adults and children, including:
- Harm to an unborn baby. Females who are able to get pregnant:
- Must use effective birth control during treatment with Corlanor.
- Tell your doctor right away if you become pregnant during treatment with Corlanor.
- Increased risk of irregular or rapid heartbeat (atrial fibrillation or heart rhythm problems). Tell your doctor if you feel any of the following symptoms of an irregular or rapid heartbeat:
- heart is pounding or racing (palpitations).
- chest pressure.
- worsened shortness of breath.
- near fainting or fainting.
- Slower than normal heart rate (bradycardia). Tell your doctor if you have:
- a slowing of heart rate, or
- symptoms of a slow heart rate such as dizziness, fatigue, lack of energy. In young children signs and symptoms of slow heart rate may include: poor feeding, difficulty breathing or turning blue.
Who should not take Corlanor?
Do not take Corlanor if you have:
- symptoms of heart failure that recently worsened
- very low blood pressure (hypotension)
- certain heart conditions: sick sinus syndrome, sinoatrial block, or 3rd degree atrioventricular block
- a slow resting heart rate before treatment with Corlanor. Ask your doctor what a slow resting heart rate is for you.
- certain liver problems
- been prescribed any medicines that can increase the effects of Corlanor.
Ask your doctor if you are not sure if you have any of the medical conditions listed above.
What should I tell my healthcare provider before taking Corlanor?
Before you take Corlanor, tell your doctor about all of your medical conditions, including if you:
- have any other heart problems, including heart rhythm problems, a slow heart rate, or a heart conduction problem.
- are breastfeeding or planning to breastfeed. It is not known if Corlanor passes into breast milk. You and your doctor should decide if you will take Corlanor or breastfeed; do not do both.
- are pregnant or planning to become pregnant. See “What is the most important information I should know about Corlanor? – Harm to an unborn baby” section.
Tell your doctor about all the medicines you take, including prescription and over the counter medicines, vitamins, and herbal supplements. Corlanor may affect the way other medicines work, and other medicines may affect how Corlanor works. This could cause serious side effects.
How should I take Corlanor?
- Take Corlanor exactly as your doctor tells you.
- Do not stop taking Corlanor without talking with your doctor.
- Corlanor comes as a tablet or as an oral solution.
- Tell your doctor if you have trouble swallowing tablets.
- Your doctor may change your dose of Corlanor during treatment
- If you are prescribed Corlanor oral solution, see the Instructions for Use that comes with your medicine for important information about how to prepare, and give or take a dose of Corlanor oral solution.
- Take Corlanor 2 times each day with food.
- If you miss a dose of Corlanor, do not give another dose. Give the next dose at the usual time.
- If you or your child take too much Corlanor, call your doctor or go to the nearest emergency room right away.
What should I avoid while taking Corlanor?
- Avoid drinking grapefruit juice and taking St. John’s wort during treatment with Corlanor. These can affect the way Corlanor works and may cause serious side effects.
What are the possible side effects of Corlanor?
Corlanor may cause serious side effects. See “What is the most important information I should know about Corlanor?”
The most common side effects of Corlanor are:
- increased blood pressure
- temporary brightness in part of your field of vision. This is usually caused by sudden changes in light (luminous phenomena). This brightness usually happens within the first 2 months of treatment with Corlanor and may go away during or after treatment with Corlanor. Be careful when driving or operating machinery where sudden changes in light can happen, especially when driving at night.
These are not all the side effects of Corlanor. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Corlanor
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Corlanor for a condition for which it was not prescribed. Do not give Corlanor to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information about Corlanor that is written for health professionals.
How should I store Corlanor?
- Store Corlanor at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Corlanor ampules in the unopened foil pouch until ready to use, to protect from light.
- Do not save or reuse any leftover Corlanor oral solution. Corlanor oral solution is sterile and does not contain a preservative.
Keep Corlanor and all medicines out of the reach of children.
What are the ingredients in Corlanor?
Active ingredient: ivabradine
Inactive ingredients:
Tablet: colloidal silicon dioxide, glycerol, hypromellose, lactose monohydrate, magnesium stearate, maize starch, maltodextrin, polyethylene glycol 6000, red iron oxide, titanium dioxide, yellow iron oxide
Oral Solution: Maltitol and water
For more information, go to www.Corlanor.com or call 1-800-772-6436.
Instructions for use for Corlanor
Corlanor (core lan ore) (ivabradine)
oral solution
Ampule
Important
Before you use Corlanor oral solution, read this important information.
Using
- These instructions are for use by adults who are preparing and taking Corlanor or giving Corlanor to an adult or child who cannot swallow tablets.
- When you receive your Corlanor oral solution carton, always check to see that the name “Corlanor” is on it and that the expiration date on the carton has not passed. Do not use an ampule of Corlanor after the expiration date on the carton.
- Corlanor oral solution comes in an ampule that contains 5 mL of medicine.
- The dose may be higher or lower than 1 ampule. To prepare a dose, you may need to use only part of an ampule or more than 1 ampule of Corlanor.
- Corlanor should only be given by an adult. A child should not give or take a dose of Corlanor by themselves.
Storing
- Store Corlanor oral solution at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Corlanor oral solution ampules in their unopened child-resistant foil pouch until ready to use, to protect from light.
- Keep Corlanor and all medicines out of the reach of children.
- Throw away any unused Corlanor oral solution right away after measuring the dose using the oral syringe. Do not save or reuse any leftover Corlanor oral solution. Corlanor oral solution does not contain a preservative.
Measuring
- Your pharmacist should provide you with an oral syringe to measure and give the prescribed dose. Always use the oral syringe given to you by your pharmacist to measure the prescribed dose. Call your pharmacist if you have not been given an oral syringe.
- If you have any questions, ask your or your child’s healthcare provider or pharmacist to show you how to measure and give the prescribed dose of Corlanor oral solution.
For more information or help, go to www.Corlanor.com or call 1-800-722-6436.
Supplies needed to take or give a dose of Corlanor
From the pharmacist

1 reusable medicine cup
Measure the dose with the oral syringe. Do not measure the dose with the medicine cup.
Do not throw away the medicine cup.
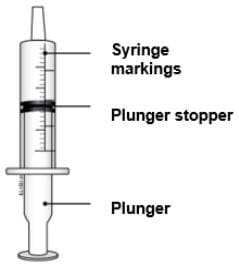
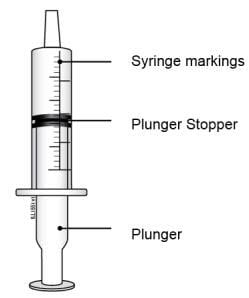
1 reusable oral syringe 1 reusable oral syringe
Oral syringes come in different sizes (Example: 0.5 mL, 1 mL, 3 mL, 5 mL, 10 mL).
Your pharmacist will give you the correct syringe depending on the dose.
If you do not receive an oral syringe and medicine cup from your pharmacist, or if you have any questions about your oral syringe, call your healthcare provider or pharmacist.
Do not throw away the oral syringe unless you can no longer clearly see the markings. If you are not sure what to do, ask your healthcare provider or pharmacist.
The carton

The carton contains 28 ampules in individual foil pouches.
Each foil pouch contains 1 ampule of Corlanor oral solution. Each foil pouch contains 1 ampule of Corlanor oral solution.
Step 1: Prepare
A. Check your prescription.
- If the prescribed dose is 5 mL or less, you will use 1 ampule pouch. If the prescribed dose is more than 5 mL, you will use 2 ampule pouches.
- Once you have opened an ampule, do not save unused Corlanor oral solution for later use.
B. Gather the supplies you will need.
- 1 or 2 Corlanor oral solution foil pouches
- 1 oral syringe (provided by the pharmacist)
- 1 medicine cup (provided by the pharmacist)
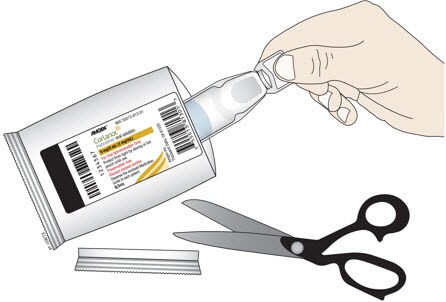
C. Cut open the foil pouch and remove the ampule. Be careful not to cut the ampule inside. Repeat with another foil pouch if you need more than 1 ampule for the prescribed dose.
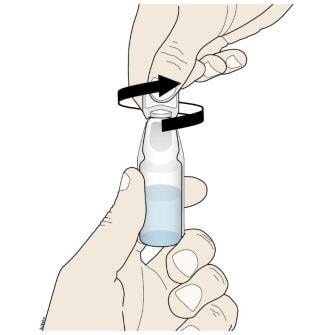
Step 2: Empty
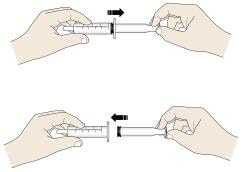
D. To open the ampule, twist the plastic top in either direction.
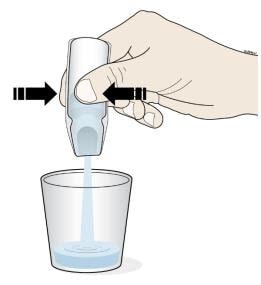
- Turn the ampule(s) upside down and squeeze to empty all of the Corlanor oral solution into the medicine cup.
- Repeat Step D if more than 1 ampule of Corlanor is needed for a prescribed dose.
Important:
- Throw away the ampules and plastic tops in household trash.
Step 3: Check
E. Check the dose in milliliters (mL) prescribed by your healthcare provider. Then find that number on the oral syringe. Your syringe and dose may look different from the example shown here.
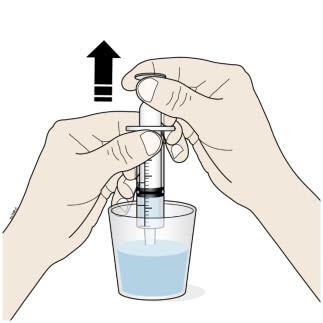
Step 4: Measure
F. Insert the tip of the oral syringe into the oral solution and slowly pull up on the plunger until the plunger stopper lines up with the syringe marking for the prescribed dose.
- Do not pull the plunger all the way out.
- Avoid pulling bubbles into the oral syringe.
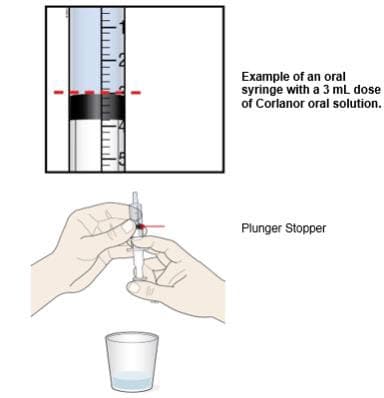
G. Turn the oral syringe right side up. Hold the syringe over the medicine cup to catch any Corlanor that might drip.
H. Adjust the amount of the Corlanor oral solution in the syringe if needed to match your prescribed dose:
- Gently press up on the plunger until the top edge of the plunger stopper lines up with the mL marking on the syringe that matches the prescribed dose.
- If you see air bubbles in the oral syringe, empty the oral solution back into the medicine cup. Repeat Steps F through H.
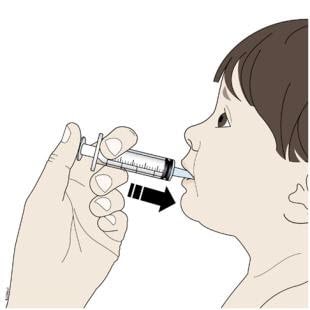
Step 5: Give
I. Place the tip of the oral syringe into your or your child’s mouth and towards the cheek.
Then slowly push down on the plunger until the oral syringe is empty.
Important:
If you miss a dose of Corlanor, do not give another dose. Give the next dose at the usual time.
Step 6: Finish
J. Rinse the reusable oral syringe and reusable medicine cup for the next dose.
- Remove the plunger from the oral syringe. Rinse the inside and outside of the oral syringe and the plunger well, with warm running water.
- Rinse the medicine cup with warm, running water.
- Place the clean oral syringe parts and medicine cup on a clean paper towel to dry.
- After the syringe parts and medicine cup dry, put the oral syringe together so that it is ready for the next use.
Label
PRINCIPAL DISPLAY PANEL – 5 MG TABLET BOTTLE LABEL
- AMGEN®
- NDC 55513-800-60
- 5
mg - Corlanor®
(ivabradine) tablets - Each tablet contains 5 mg ivabradine equivalent
to 5.39 mg ivabradine as hydrochloride.
Oral Use.
Store at 25°C (77°F); excursions permitted to
15°C to 30°C (between 59°F to 86°F)
[See USP for controlled room temperature].
Keep out of the sight and reach of children.
Dispense the enclosed Medication
Guide to each patient. - Rx Only
- 60
Film-coated
tablets

PRINCIPAL DISPLAY PANEL – 7.5 MG TABLET BOTTLE LABEL
- AMGEN®
- NDC 55513-810-60
- 7.5
mg - Corlanor®
(ivabradine) tablets - Each tablet contains 7.5 mg ivabradine equivalent
to 8.085 mg ivabradine as hydrochloride.
Oral Use.
Store at 25°C (77°F); excursions permitted to
15°C to 30°C (between 59°F to 86°F)
[See USP for controlled room temperature].
Keep out of the sight and reach of children.
Dispense the enclosed Medication
Guide to each patient. - Rx Only
- 60
Film-coated
tablets

SRC: NLM .
