Caprelsa
Generic name: vandetanib
Drug classes: EGFR inhibitors, Multikinase inhibitors, VEGF/VEGFR inhibitors
Medically reviewed by A Ras MD.
What is Caprelsa?
Caprelsa is a prescription medicine used to treat medullary thyroid cancer that cannot be removed by surgery or that has spread to other parts of the body. It takes a long time to get rid of Caprelsa from your body and you may be at risk for side effects related to Caprelsa after you have stopped your treatment.
It is not known if Caprelsa is safe and effective in children.
Description
Vandetanib has the chemical name N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-methylpiperidin-4-yl) methoxy]quinazolin-4-amine.
The structural and molecular formulas are:
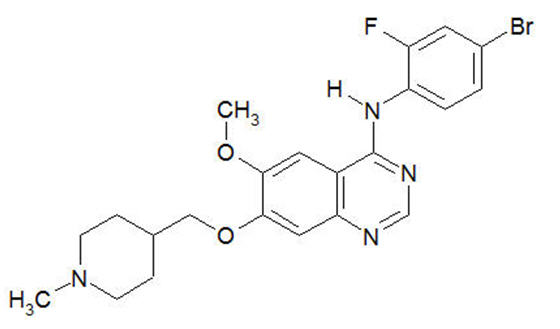
C22H24BrFN4O2
Vandetanib has a molecular weight of 475.36. Vandetanib exhibits pH-dependent solubility, with increased solubility at lower pH. Vandetanib is practically insoluble in water with a value of 0.008 mg/mL at 25°C (77°F).
CAPRELSA tablets for daily oral administration are available in two dosage strengths containing either 100 mg or 300 mg of vandetanib. The tablet cores contain the following inactive ingredients: calcium hydrogen phosphate dihydrate, microcrystalline cellulose, crospovidone, povidone, and magnesium stearate. The tablet film-coat contains the following inactive ingredients: hypromellose 2910, macrogol 300, and titanium dioxide E171.
Mechanism of Action
In vitro studies have shown that vandetanib inhibits the tyrosine kinase activity of the EGFR and VEGFR families, RET, BRK, TIE2, and members of the EPH receptor and Src kinase families. These receptor tyrosine kinases are involved in both normal cellular function and pathologic processes such as oncogenesis, metastasis, tumor angiogenesis, and maintenance of the tumor microenvironment. In addition, the N-desmethyl metabolite of the drug, representing 7 to 17.1% of vandetanib exposure, has similar inhibitory activity to the parent compound for VEGF receptors (KDR and Flt-1) and EGFR.
In vitro, vandetanib inhibited epidermal growth factor (EGF)-stimulated receptor tyrosine kinase phosphorylation in tumor cells and endothelial cells and VEGF-stimulated tyrosine kinase phosphorylation in endothelial cells.
In vivo, vandetanib administration reduced tumor cell-induced angiogenesis, tumor vessel permeability, and inhibited tumor growth and metastasis in mouse models of cancer.
What is the most important information I should know about Caprelsa?
Caprelsa can cause a change in the electrical activity of your heart called QT prolongation, which can cause irregular heartbeats and that may lead to death. You should not take Caprelsa if you have had a condition called long QT syndrome since birth.
Your healthcare provider should perform tests to check the levels of your blood potassium, calcium, magnesium, and thyroid-stimulating hormone (TSH) as well as the electrical activity of your heart with a test called an electrocardiogram (ECG). You should have these tests:
- Before starting Caprelsa
- Regularly during Caprelsa treatment:
- 2 to 4 weeks after starting Caprelsa
- 8 to 12 weeks after starting Caprelsa
- every 3 months thereafter
- if your healthcare provider changes your dose of Caprelsa
- if you start taking medicine that causes QT prolongation
- s instructed by your healthcare provider
Your healthcare provider may stop your Caprelsa treatment for a while and restart you at a lower dose if you have QT prolongation.
Call your healthcare provider right away if you feel faint, light-headed, or feel your heart beating irregularly while taking Caprelsa. These may be symptoms related to QT prolongation.
Who should not take Caprelsa?
Do not take Caprelsa if you have had QT prolongation.
What should I tell my healthcare provider before taking Caprelsa?
Before you take Caprelsa, tell your healthcare provider if you:
- have any heart problems, including a condition called congenital long QT syndrome
- have an irregular heartbeat
- take or have stopped taking a medicine that causes QT prolongation
- have low blood levels of potassium, calcium, or magnesium
- have high blood levels of thyroid-stimulating hormone
- have high blood pressure
- have skin problems
- have a history of breathing problems
- have a recent history of coughing up blood or bleeding
- have diarrhea
- have liver problems
- have kidney problems
- have seizures or are being treated for seizures
- are pregnant or plan to become pregnant. Caprelsa can cause harm to your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- If you are able to become pregnant, you should use effective birth control during your treatment with Caprelsa and for at least 4 months after your last dose of Caprelsa.
- Talk to your healthcare provider about birth control methods to prevent pregnancy while you are taking Caprelsa.
- are breastfeeding or plan to breastfeed. It is not known if Caprelsa passes into your breast milk. You and your healthcare provider should decide if you will take Caprelsa or breastfeed. You should not do both.
- plan to have surgery or have had a recent surgery. You should stop taking Caprelsa at least 1 month before planned surgery. See “What are the possible side effects of Caprelsa?”
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Caprelsa and other medicines may affect each other causing side effects.
Especially tell your healthcare provider if you take:
- St. John’s Wort. You should not take St. John’s Wort while taking Caprelsa
- certain medicines that can affect how your liver breaks down medicine
- a medicine for your heart
Ask your healthcare provider if you are not sure if your medicine is one listed above.
Do not take other medicines while taking Caprelsa until you have talked with your healthcare provider or pharmacist.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Caprelsa?
- Take Caprelsa exactly as your healthcare provider tells you to take it. Do not change your dose or stop taking Caprelsa unless your healthcare provider tells you to.
- Caprelsa may be taken with or without food.
- Swallow Caprelsa tablets whole with water.
- Do not crush or chew Caprelsa tablets. If Caprelsa tablets are accidentally crushed, contact with skin should be avoided. If contact occurs, wash affected areas well with water.
- If you cannot swallow Caprelsa tablets whole:
- place your dose of Caprelsa in a glass that contains 2 ounces of noncarbonated water (no other liquids should be used).
- stir the Caprelsa tablet(s) and water mixture for about 10 minutes or until the tablet(s) are in very small pieces (the tablets will not completely dissolve).
- swallow Caprelsa and water mixture right away.
- if any Caprelsa and water mixture remains in the glass, mix with an additional 4 ounces of noncarbonated water and swallow the mixture to make sure that you take your full dose of Caprelsa.
- If you miss a dose and your next dose is in:
- less than 12 hours, take your next dose at the normal time. Do not make up for the missed dose.
- 12 hours or more, take the missed dose as soon as you remember. Take the next dose at the normal time.
Call your healthcare provider right away if you take too much Caprelsa.
- During treatment with Caprelsa, your healthcare provider should check your blood and heart for side effects. See “What is the most important information I should know about Caprelsa?”
- Your healthcare provider should check your blood pressure regularly during your treatment with Caprelsa.
What should I avoid while taking Caprelsa?
- Limit exposure to the sun. Caprelsa can make your skin sensitive to the sun. While taking Caprelsa and for 4 months after stopping your Caprelsa treatment, use sun block and wear clothes that cover your skin, including your head, arms and legs when you go outdoors.
- Use caution before driving or using machinery. Keep in mind Caprelsa may make you feel tired, weak, or cause blurred vision.
What are the possible side effects of Caprelsa?
Caprelsa may cause serious side effects, including:
- See “What is the most important information I should know about Caprelsa?”
- Serious skin reactions. Caprelsa can cause serious skin reactions such as toxic epidermal necrolysis and Stevens-Johnson syndrome, or other serious skin reactions that may affect any part of your body. These serious skin reactions may be life threatening and you may need to be treated in a hospital. Call your healthcare provider right away if you experience any of these symptoms.
- Skin rash or acne
- Dry skin
- Itching
- Blisters on your skin
- Blisters or sores in your mouth
- Peeling of your skin
- Fever
- Muscle or joint aches
- Redness or swelling of your face, hands, or soles of your feet
- Breathing problems (interstitial lung disease). Caprelsa may cause a breathing problem called interstitial lung disease that can lead to death. Tell your healthcare provider right away if you experience sudden or worsening shortness of breath or cough.
- Stroke. Strokes have been reported in some people who have taken Caprelsa and in some cases have caused death. Stop taking Caprelsa and call your healthcare provider right away if you have symptoms of a stroke which may include:
- numbness or weakness of the face, arm or leg, especially on one side of the body
- sudden confusion, trouble speaking or understanding
- sudden trouble seeing in one or both eyes
- sudden trouble walking, dizziness, loss of balance or coordination
- sudden, severe headache
- Bleeding. Bleeding can happen during your treatment with Caprelsa. Tell your healthcare provider right away if you have severe bleeding while you are taking Caprelsa.
- Heart failure. Caprelsa can cause heart failure that can lead to death. You may have to stop taking Caprelsa if you have heart failure. Heart failure may not be reversible after stopping Caprelsa. Your healthcare provider should monitor you for signs and symptoms of heart failure.
- Diarrhea. Diarrhea is often a symptom of medullary thyroid cancer. Caprelsa can also cause diarrhea or make diarrhea worse. Your healthcare provider should check your blood levels to monitor your electrolytes more frequently if you have diarrhea.
- Thyroid hormones. You can have changes in your thyroid hormone when taking Caprelsa. Your healthcare provider should monitor your thyroid hormone levels while taking Caprelsa.
- High blood pressure (hypertension). If you develop high blood pressure or your high blood pressure gets worse, your healthcare provider may lower your dose of Caprelsa or tell you to stop taking Caprelsa until your blood pressure is under control. Your healthcare provider may prescribe another medicine to control your high blood pressure.
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS). A condition called reversible posterior leukoencephalopathy syndrome can happen while taking Caprelsa. Call your healthcare provider right away if you have:
- headaches
- seizures
- confusion
- changes in vision
- problems thinking
- Possible wound healing problems. Wounds may not heal properly during Caprelsa treatment. Tell your healthcare provider if you plan to have any surgery before starting or during treatment with Caprelsa.
- You should stop taking Caprelsa at least 1 month before planned surgery.
- Your healthcare provider should tell you when you may start taking Caprelsa again after surgery.
The most common side effects of Caprelsa include:
- diarrhea
- rash
- acne
- nausea
- high blood pressure
- headache
- feeling tired
- loss of appetite
- upper respiratory tract infections
- stomach (abdominal) pain
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Caprelsa. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Caprelsa
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Caprelsa for a condition for which it was not prescribed. Do not give Caprelsa to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes important information about Caprelsa. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Caprelsa that is written for health professionals. For more information, go to www.caprelsa.com or call 1-800-817-2722.
How should I store Caprelsa?
- Store Caprelsa tablets at 59°F to 86°F (15°C to 30°C).
- Safely throw away medicine that is out of date or that you no longer need. Ask your pharmacist how to safely throw away Caprelsa tablets.
Keep Caprelsa and all medicines out of the reach of children.
What are the ingredients in Caprelsa?
Active ingredient: vandetanib
Inactive ingredients:
- Tablet core: calcium hydrogen phosphate dihydrate, microcrystalline cellulose, crospovidone, povidone, and magnesium stearate
- Tablet film-coat: hypromellose 2910, macrogol 300, and titanium dioxide E171
Label
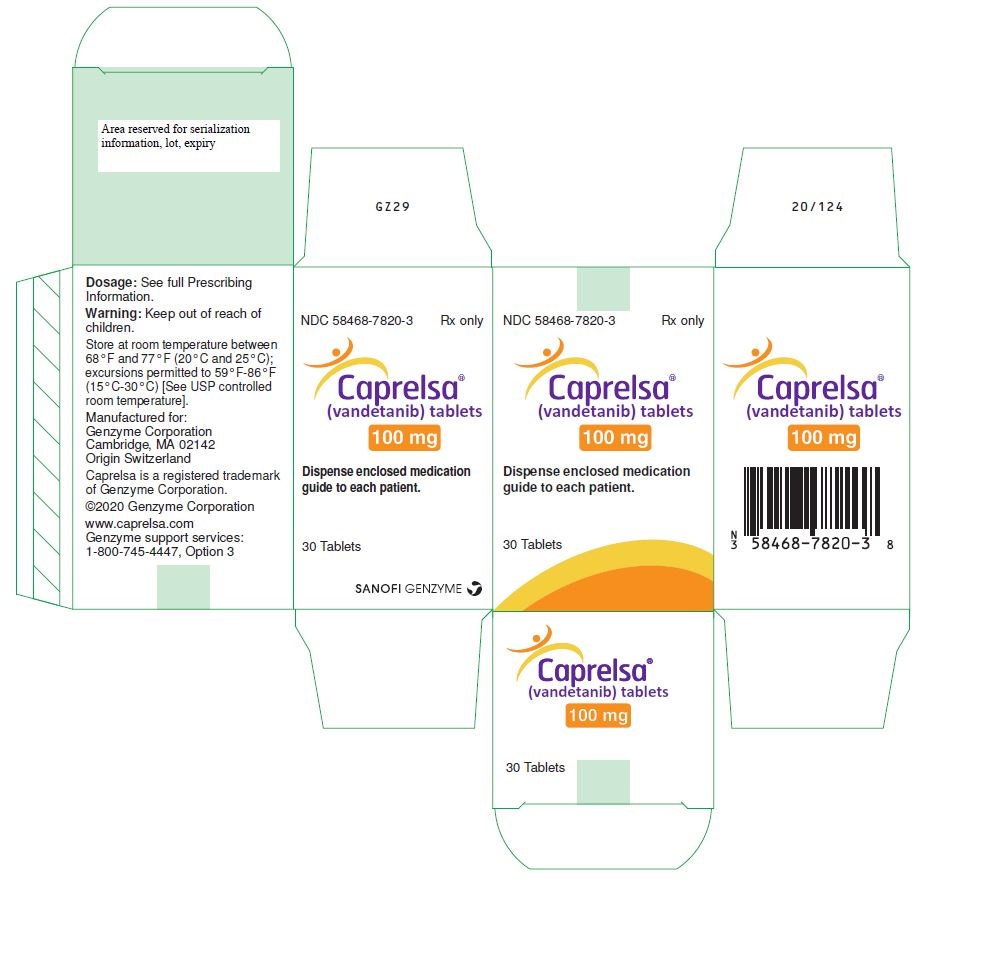
PRINCIPAL DISPLAY PANEL – 100 MG TABLET BOTTLE CARTON
- NDC 58468-7820-3 Rx only
- Caprelsa®
(vandetanib) tablets - 100 mg
- Dispense enclosed medication
guide to each patient. - 30 Tablets
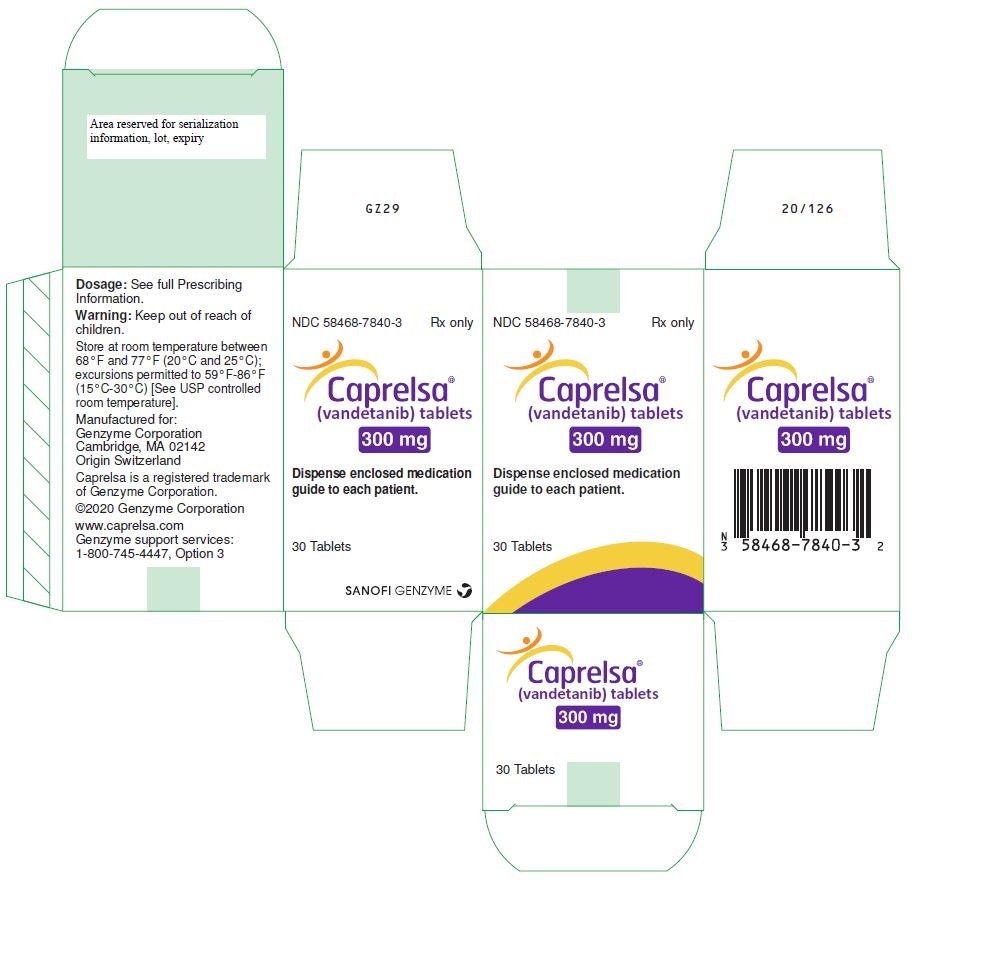
PRINCIPAL DISPLAY PANEL – 300 MG TABLET BOTTLE CARTON
- NDC 58468-7840-3 Rx only
- Caprelsa®
(vandetanib) tablets - 300 mg
- Dispense enclosed medication
guide to each patient. - 30 Tablets
SRC: NLM .

