Belbuca
Generic name: buprenorphine (oral/buccal)
Drug class: Narcotic analgesics
Medically reviewed by A Ras MD.
What is Belbuca?
Belbuca is a strong prescription pain medicine that contains an opioid (narcotic) that is used to manage pain severe enough to require daily around-the-clock, long-term treatment with an opioid, when other pain treatments such as non-opioid pain medicines or immediate-release opioid medicines do not treat your pain well enough or you cannot tolerate them.
A long-acting opioid pain medicine that can put you at risk for overdose and death. Even if you take your dose correctly as prescribed, you are at risk for opioid addiction, abuse, and misuse that can lead to death.
Not for use to treat pain that is not around-the-clock.
Description
BELBUCA is a buccal film that provides transmucosal delivery of buprenorphine, a partial opioid agonist. BELBUCA is a rectangular bi-layer, peppermint-flavored, buccal film with rounded corners, consisting of a white to off-white backing layer with strength identifier printed in black ink and a light yellow to yellow active mucoadhesive layer containing buprenorphine hydrochloride. The yellow side of the film is applied to the inside of the cheek where it adheres to the moist buccal mucosa to deliver the drug as the film dissolves.
Buprenorphine hydrochloride USP is the active ingredient in BELBUCA. The chemical name of buprenorphine hydrochloride is 6,14-ethenomorphinan-7-methanol, 17-(cyclopropylmethyl)- α-(1,1-dimethylethyl)-4, 5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-, hydrochloride, [5α, 7α, (S)]. Its structural formula is as follows:
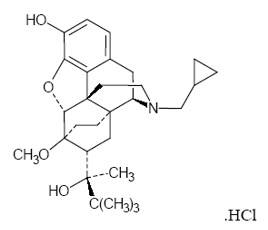
The molecular weight of buprenorphine hydrochloride is 504.10; the empirical formula is C29H41NO4∙HCl. Buprenorphine hydrochloride occurs as a white or off-white crystalline powder. It is sparingly soluble in water, freely soluble in methanol, soluble in alcohol, and practically insoluble in cyclohexane. The pKa is 8.5 for the amine function and 10.0 for the phenol function.
Dosage strengths of BELBUCA are based on the active moiety, buprenorphine. BELBUCA is available as 75 mcg, 150 mcg, 300 mcg, 450 mcg, 600 mcg, 750 mcg, and 900 mcg buprenorphine per film. The strength of each film is dependent on the buprenorphine concentration in the formulation and the surface area of the film. Table 6 lists the dosage strength, equivalent amount of buprenorphine hydrochloride USP (active ingredient), unique identifier and film size for each strength.
| Buprenorphine Strength (mcg) | Buprenorphine Hydrochloride (mcg) | BELBUCA Identifier |
Film Size (cm2) |
|---|---|---|---|
| 75 | 80.9 | E0 | 1.215 |
| 150 | 161.8 | E1 | 2.431 |
| 300 | 323.4 | E3 | 0.934 |
| 450 | 485.1 | E4 | 1.400 |
| 600 | 646.8 | E6 | 1.867 |
| 750 | 808.5 | E7 | 2.334 |
| 900 | 970.2 | E9 | 2.801 |
Each buccal film also contains carboxymethylcellulose sodium USP, citric acid anhydrous USP, hydroxyethylcellulose NF, hydroxypropylcellulose NF, methylparaben NF, monobasic sodium phosphate anhydrous USP, peppermint oil NF, polycarbophil USP, propylene glycol USP, propylparaben NF, sodium benzoate NF, sodium hydroxide NF, saccharin sodium NF, titanium dioxide USP, vitamin E acetate USP, yellow iron oxide, purified water USP, and TekPrint SW-9008 black ink (shellac NF, black iron oxide NF).\
Mechanism of Action
Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptor.
What is the most important information I should know about Belbuca?
- Get emergency help right away if you take too much Belbuca (overdose). When you first start taking Belbuca, when your dose is changed, or if you take too much (overdose), serious or life-threatening breathing problems that can lead to death may occur.
- Taking Belbuca with other opioid medicines, benzodiazepines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, decreased awareness, breathing problems, coma, and death.
- Never give anyone else your Belbuca. They could die from taking it. Selling or giving away Belbuca is against the law.
- Store Belbuca securely, out of sight and reach of children, and in a location not accessible by others, including visitors to the home.
Who should not take Belbuca?
Do not use Belbuca if you have:
- severe asthma, trouble breathing, or other lung problems.
- a bowel blockage or have narrowing of the stomach or intestines.
What should I tell my healthcare provider before taking Belbuca?
Before applying Belbuca, tell your healthcare provider if you have a history of:
- head injury, seizures
- liver, kidney, thyroid problems
- problems urinating
- heart rhythm problems (long QT syndrome)
- pancreas or gallbladder problems
- abuse of street or prescription drugs, alcohol addiction, or mental health problems
Tell your healthcare provider if you are:
- pregnant or planning to become pregnant. Prolonged use of Belbuca during pregnancy can cause withdrawal symptoms in your newborn baby that could be life-threatening if not recognized and treated.
- breastfeeding. Not recommended during treatment with Belbuca. It may harm your baby.
- taking prescription or over-the-counter medicines, vitamins, or herbal supplements. Taking Belbuca with certain other medicines can cause serious side effects and could lead to death.
How should I take Belbuca?
When taking Belbuca:
- Do not change your dose. Apply Belbuca exactly as prescribed by your healthcare provider. Use the lowest effective dose possible for the shortest time needed.
- See the instructions for use that comes with Belbuca.
- Do not apply Belbuca if the package seal is broken or the film is cut, damaged, or changed in any way.
- After the film has adhered to your cheek, avoid eating or drinking until the film has completely dissolved, usually within 30 minutes.
- Avoid touching or moving the buccal film with your tongue or fingers.
- Do not chew, swallow, snort or inject Belbuca. This will result in uncontrolled delivery of buprenorphine and may cause you to overdose and die.
- Call your healthcare provider if the dose you are using does not control your pain.
- Do not stop using Belbuca without talking to your healthcare provider.
- Dispose of expired, unwanted, or unused Belbuca by removing the Belbuca film from the foil packaging, and promptly flushing down the toilet (if a drug takeback option is not readily available). Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
What should I avoid while taking Belbuca?
While using Belbuca do not:
- Drive or operate heavy machinery, until you know how Belbuca affects you. Belbuca can make you sleepy, dizzy, or lightheaded.
- Drink alcohol or use prescription or over-the-counter medicines containing alcohol. Using products containing alcohol during treatment with Belbuca may cause you to overdose and die.
What are the possible side effects of Belbuca?
The possible side effects of Belbuca are:
- nausea, constipation, headache, vomiting, dizziness, and sleepiness. Call your healthcare provider if you have any of these symptoms and they are severe.
Get emergency medical help if you have:
- trouble breathing, shortness of breath, fast heartbeat, chest pain, swelling of your face, tongue or throat, extreme drowsiness, light-headedness when changing positions, feeling faint, agitation, high body temperature, trouble walking, stiff muscles, or mental changes such as confusion.
These are not all the possible side effects of Belbuca. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Belbuca?
Store at 20°C to 25°C (68°F to 77°F), with excursions permitted between 15°C and 30°C (59°F and 86°F).
Store Belbuca securely and dispose of properly.
Keep out of reach of children.
What are the ingredients in Belbuca?
Active ingredients: buprenorphine hydrochloride
Inactive ingredients: hydroxyethyl cellulose, titanium dioxide, peppermint oil, propylene glycol, sodium benzoate, methylparaben, propylparaben, ferric oxide yellow, anhydrous citric acid, alpha-tocopherol acetate, sodium phosphate monobasic anhydrous, sodium hydroxide, polycarbophil, hydroxypropyl cellulose, carboxymethylcellulose sodium, saccharin sodium.
Label
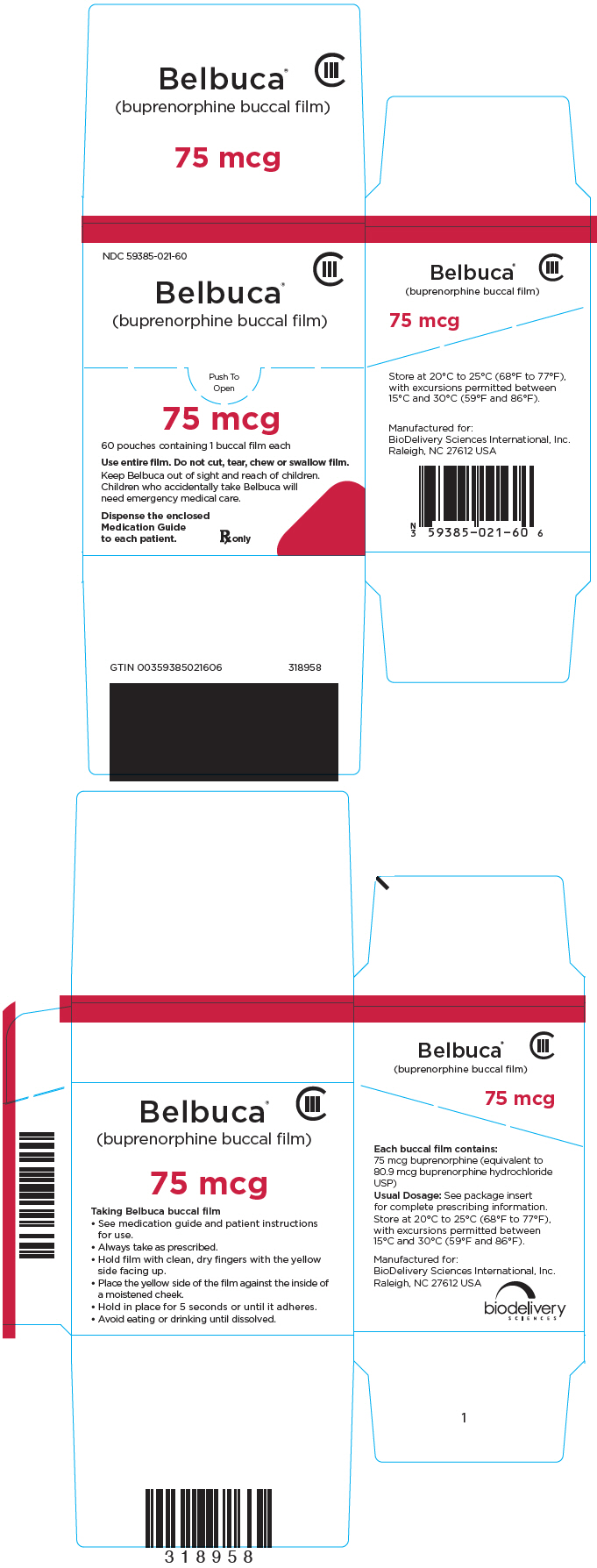
PRINCIPAL DISPLAY PANEL – 75 MCG FILM POUCH BOX
- NDC 59385-021-60
- CIII
- Belbuca®
(buprenorphine buccal film) - Push To
Open - 75 mcg
- 60 pouches containing 1 buccal film each
- Use entire film. Do not cut, tear, chew or swallow film.
- Keep Belbuca out of sight and reach of children.
Children who accidentally take Belbuca will
need emergency medical care. - Dispense the enclosed
Medication Guide
to each patient.
Rx only
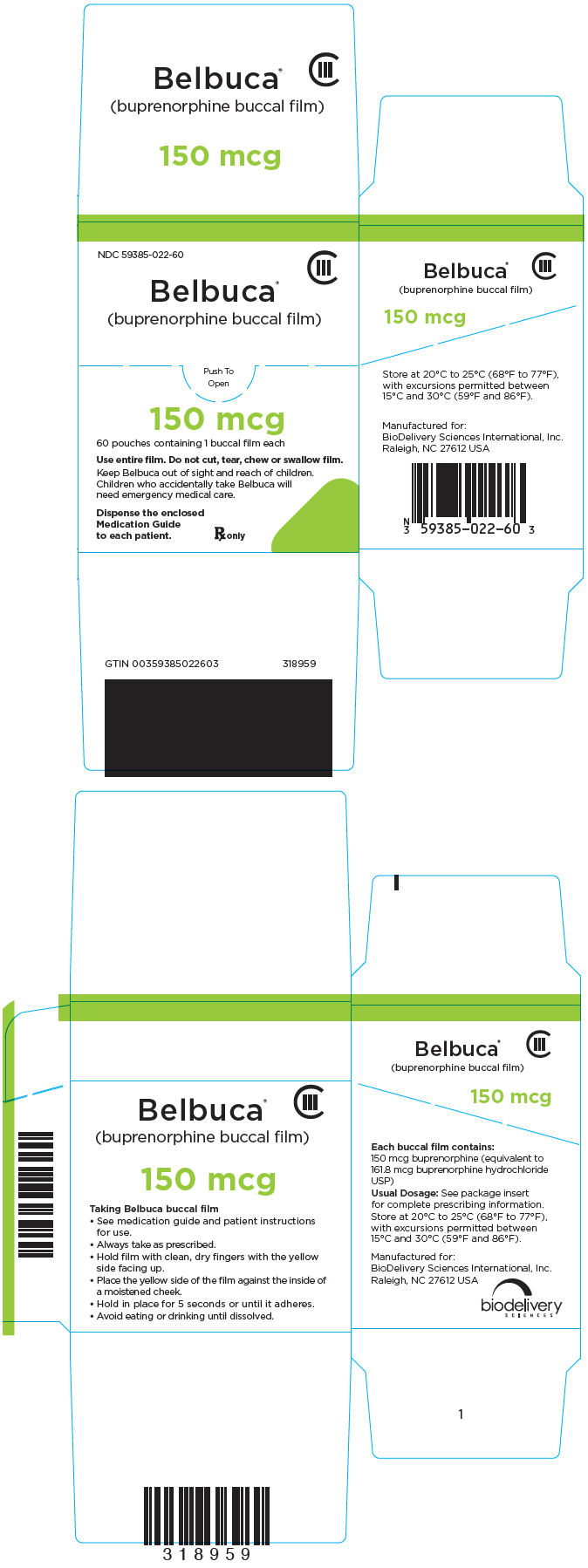
PRINCIPAL DISPLAY PANEL – 150 MCG FILM POUCH BOX
- NDC 59385-022-60
- CIII
- Belbuca®
(buprenorphine buccal film) - Push To
Open - 150 mcg
- 60 pouches containing 1 buccal film each
- Use entire film. Do not cut, tear, chew or swallow film.
- Keep Belbuca out of sight and reach of children.
Children who accidentally take Belbuca will
need emergency medical care. - Dispense the enclosed
Medication Guide
to each patient. - Rx only
SRC: NLM .
