Zynlonta
Generic name: loncastuximab tesirine-lpyl
Dosage form: lyophilized powder for injection, for intravenous use
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Zynlonta?
Zynlonta is a prescription medicine used to treat adults with certain types of large B-cell lymphoma that has come back (relapsed) or that did not respond to previous treatment (refractory), who have already received two or more treatments for their cancer.
It is not known if Zynlonta is safe and effective in children.
Description
Loncastuximab tesirine-lpyl is a CD19-directed antibody and alkylating agent conjugate, consisting of a humanized IgG1 kappa monoclonal antibody conjugated to SG3199, a pyrrolobenzodiazepine (PBD) dimer cytotoxic alkylating agent, through a protease-cleavable valine-alanine linker. SG3199 attached to the linker is designated as SG3249, also known as tesirine.
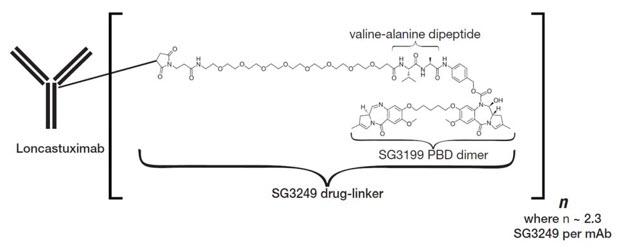
Loncastuximab tesirine-lpyl has an approximate molecular weight of 151 kDa. An average of 2.3 molecules of SG3249 are attached to each antibody molecule. Loncastuximab tesirine-lpyl is produced by chemical conjugation of the antibody and small molecule components. The antibody is produced by mammalian (Chinese hamster ovary) cells, and the small molecule components are produced by chemical synthesis.
ZYNLONTA (loncastuximab tesirine-lpyl) for injection is supplied as a sterile, white to off-white, preservative-free, lyophilized powder, which has a cake-like appearance, for intravenous infusion after reconstitution and dilution. Each single-dose vial delivers 10 mg of loncastuximab tesirine-lpyl, L-histidine (2.8 mg), L-histidine monohydrochloride (4.6 mg), polysorbate 20 (0.4 mg), and sucrose (119.8 mg). After reconstitution with 2.2 mL Sterile Water for Injection, USP, the final concentration is 5 mg/mL with a pH of approximately 6.0.
Mechanism of Action
Loncastuximab tesirine-lpyl is an antibody-drug conjugate (ADC) targeting CD19. The monoclonal IgG1 kappa antibody component binds to human CD19, a transmembrane protein expressed on the surface of cells of B-lineage origin. The small molecule component is SG3199, a PBD dimer and alkylating agent.
Upon binding to CD19, loncastuximab tesirine-lpyl is internalized followed by release of SG3199 via proteolytic cleavage. The released SG3199 binds to the DNA minor groove and forms highly cytotoxic DNA interstrand crosslinks, subsequently inducing cell death. Loncastuximab tesirine-lpyl had anticancer activity in animal models of lymphoma.
What should I tell my healthcare provider before using Zynlonta?
Before you receive Zynlonta, tell your healthcare provider about all of your medical conditions, including if you:
- have an active infection or have had one recently.
- have liver problems.
- are pregnant or plan to become pregnant. Zynlonta can harm your unborn baby.
- Females who can become pregnant:
- Your healthcare provider may do a pregnancy test before starting treatment with Zynlonta.
- You should use effective birth control (contraception) during treatment with Zynlonta and for 9 months after the last dose of Zynlonta. Talk to your healthcare provider about effective birth control. Tell your healthcare provider right away if you become pregnant or think that you are pregnant during treatment with Zynlonta.
- Males with female partners who can become pregnant:
- You should use effective birth control (contraception) during treatment with Zynlonta and for 6 months after the last dose of Zynlonta.
- Females who can become pregnant:
- are breastfeeding or plan to breastfeed. It is not known if Zynlonta passes into breast milk. Do not breastfeed during treatment with Zynlonta and for 3 months after the last dose of Zynlonta.
Tell your healthcare provider about all the medicines that you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get new medicine.
How should I use Zynlonta?
- Zynlonta is given to you by your healthcare provider as an intravenous (IV) infusion into your vein over 30 minutes.
- Zynlonta is usually given every 3 weeks.
- Your healthcare provider may give you medicine before each infusion to decrease your chance of side effects.
- Your healthcare provider may stop your treatment, delay your treatment, or change your dose of Zynlonta if you have severe side effects.
- Your healthcare provider should do blood tests regularly to check for side effects of Zynlonta.
- Your healthcare provider will decide how many treatments you need.
What should I avoid while using Zynlonta?
Avoid or limit your exposure to sunlight, including sunlight through glass, such as buildings or vehicle windows, and artificial sunlight such as sunlamps or tanning beds. Exposure to sunlight during treatment with Zynlonta can cause skin reactions or rash. Use sun protection measures such as sunscreen and wear loose-fitting clothes that cover your skin while out in sunlight.
What are the possible side effects of Zynlonta?
Zynlonta may cause serious side effects, including:
- Fluid retention. Your body may hold too much fluid during treatment with Zynlonta. This can be serious. Tell your healthcare provider if you develop new or worsening swelling or puffiness, weight gain, chest pain, shortness of breath, or trouble breathing.
- Low blood cell counts (platelets, red blood cells, and white blood cells). Low blood cell counts are common with Zynlonta but can also be serious or severe. Your healthcare provider will monitor your blood counts during treatment with Zynlonta. Tell your healthcare provider right away if you get a fever of 100.4°F (38°C) or above, or any bruising or bleeding.
- Infections. Serious infections, including infections that can cause death, have happened in people treated with Zynlonta. Tell your healthcare provider right away if you have new or worsening signs or symptoms of infection, including:
- fever
- chills
- flu-like symptoms (cough, tiredness or weakness, and body aches)
- headache
- breathing problems
- cuts or scrapes that are red, warm, swollen or painful
- Skin Reactions. Serious skin reactions have happened in people treated with Zynlonta. Tell your healthcare provider if you get new or worsening skin reactions, including sensitivity to sunlight, skin rash, peeling, redness or irritation. You may burn more easily or get severe sunburns. See “What should I avoid while recieving Zynlonta?”
The most common side effects of Zynlonta include:
- feeling tired or weak
- skin rash
- swelling
- nausea
- muscle or joint pain
- increase in blood sugar (hyperglycemia)
- changes in certain blood or laboratory tests
Zynlonta may cause fertility problems in males which may affect your ability to father children. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of Zynlonta. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Zynlonta
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider or pharmacist for information about Zynlonta that is written for healthcare professionals.
What are the ingredients in Zynlonta?
Active ingredient: loncastuximab tesirine-lpyl
Inactive ingredients: L-histidine, L-histidine monohydrochloride, polysorbate 20, and sucrose.
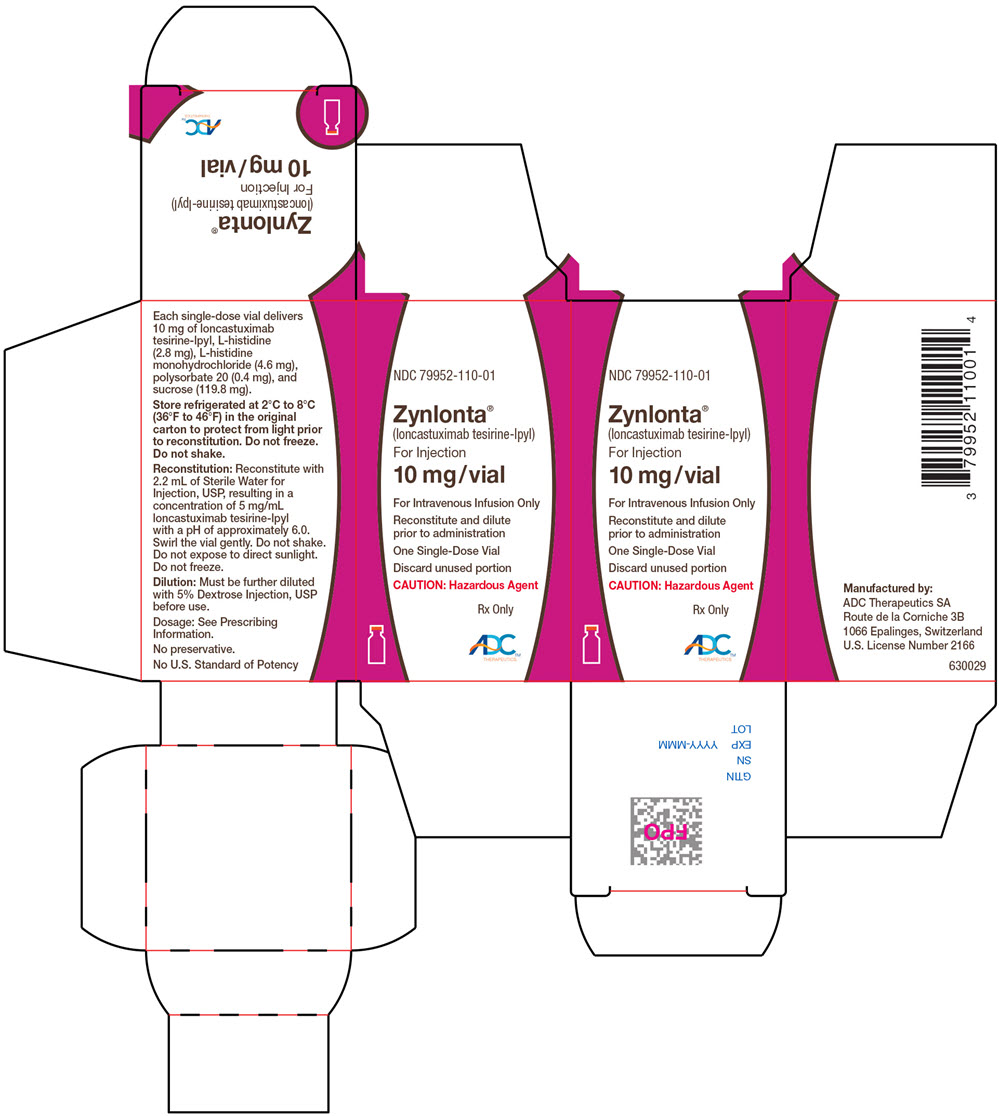
Label
PRINCIPAL DISPLAY PANEL – 10 MG VIAL CARTON
- NDC 79952-110-01
- Zynlonta™
(loncastuximab tesirine-lpyl)
For Injection - 10 mg/vial
- For Intravenous Infusion Only
- Reconstitute and dilute
prior to administration - One Single-Dose Vial
- Discard unused portion
- CAUTION: Hazardous Agent
- Rx Only
- ADC™
THERAPEUTICS
SRC: NLM .
