Zubsolv
Generic name: buprenorphine and naloxone (sublingual tablets)
Drug class: Narcotic analgesic combinations
Medically reviewed by A Ras MD.
What is Zubsolv?
Zubsolv is a prescription medicine used to treat adults who are addicted to opioid drugs (either prescription or illegal); as part of a complete treatment program that also includes counseling and behavioral therapy.
Zubsolv is a controlled substance (CIII) because it contains buprenorphine, which can be a target for people who abuse prescription medicines or street drugs.
It is not known if Zubsolv is safe or effective in children.
Description
ZUBSOLV (buprenorphine and naloxone) sublingual tablets are white menthol-flavored tablets in an oval shape for the dosage strength 0.7 mg/0.18 mg, a triangular shape for the dosage strength 1.4 mg /0.36 mg, a D shape for the dosage strength 2.9 mg/0.71 mg, a round shape for the dosage strength 5.7 mg/1.4 mg, a diamond shape for the dosage strength 8.6 mg/2.1 mg and a capsule shape for the dosage strength 11.4 mg/2.9 mg.
They are debossed with the respective dosage strength of buprenorphine. They contain buprenorphine HCl, an opioid partial agonist and naloxone HCl dihydrate, an opioid antagonist, at a ratio of 4:1 (ratio of free bases). ZUBSOLV is intended for sublingual administration and is available in six dosage strengths, 0.7 mg buprenorphine with 0.18 mg naloxone, 1.4 mg buprenorphine with 0.36 mg naloxone, 2.9 mg buprenorphine with 0.71 mg naloxone, 5.7 mg buprenorphine with 1.4 mg naloxone, 8.6 mg buprenorphine with 2.1 mg naloxone and 11.4 mg buprenorphine with 2.9 mg naloxone. Each sublingual tablet also contains mannitol, citric acid, sodium citrate, microcrystalline cellulose, croscarmellose sodium, sucralose, menthol, silicon dioxide and sodium stearyl fumarate and menthol flavor.
Chemically, buprenorphine HCl is (2S)-2-[17-(cyclopropylmethyl)-4,5α-epoxy-3-hydroxy-6-methoxy-6α,14-ethano-14α-morphinan-7α-yl]-3,3-dimethylbutan-2-ol hydrochloride. It has the following chemical structure:
![It has the following chemical structure:Chemically, buprenorphine HCl is (2S)-2-[17-(cyclopropylmethyl)-4,5α-epoxy-3-hydroxy-6-methoxy-6α,14-ethano-14α-morphinan-7α-yl]-3,3-dimethylbutan-2-ol hydrochloride.](https://dailymed.nlm.nih.gov/dailymed/image.cfm?name=zubsolv-sublingual-tablets-01.jpg&setid=5f5cfcfe-d52b-49e6-8fe4-550477332dd2)
Buprenorphine HCl has the molecular formula C29 H41 NO4 • HCl and the molecular weight is 504.10. It is a white or off-white crystalline powder, sparingly soluble in water, freely soluble in methanol, soluble in alcohol, and practically insoluble in cyclohexane.
Chemically, naloxone HCl dihydrate is 17-Allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one hydrochloride dihydrate. It has the following chemical structure:
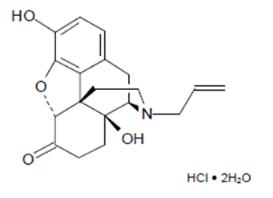
Naloxone HCl dihydrate has the molecular formula C19H21NO4 • HCl • 2H20 and the molecular weight is 399.87. It is a white to slightly off-white powder and is freely soluble in water, soluble in alcohol, and practically insoluble in toluene and ether.
Mechanism of Action
ZUBSOLV contains buprenorphine and naloxone. Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptor. Naloxone is a potent antagonist at mu-opioid receptors and produces opioid withdrawal signs and symptoms, if administered parenterally, in individuals physically dependent on full opioid agonists.
What is the most important information I should know about Zubsolv?
Important:
Keep ZUBSOLV in a secure place away from children. Accidental use by a child is a medical emergency and can result in death. If a child accidentally uses ZUBSOLV, get emergency help right away.
- Zubsolv can cause serious and life-threatening breathing problems. Call your doctor right away or get emergency help if:
- You feel faint, dizzy, or confused.
- Your breathing gets much slower than is normal for you.
These can be signs of an overdose or other serious problems.
- Do not switch from Zubsolv to other medicines that contain buprenorphine without talking with your doctor. The amount of buprenorphine in a dose of Zubsolv is not the same as the amount of buprenorphine in other medicines that contain buprenorphine. Your doctor will prescribe a starting dose of buprenorphine that may be different than other buprenorphine containing medicines you may have been taking.
- Zubsolv contains an opioid that can cause physical dependence.
- Do not stop taking Zubsolv without talking to your doctor. You could become sick with uncomfortable withdrawal signs and symptoms because your body has become used to this medicine.
- Physical dependence is not the same as drug addiction.
- Zubsolv is not for occasional or “as needed” use.
- An overdose, and even death, can happen if you take benzodiazepines, sedatives, tranquilizers, or alcohol while using Zubsolv. Ask your doctor what you should do if you are taking one of these.
- Call a doctor or get emergency help right away if you:
- Feel sleepy and uncoordinated.
- Have blurred vision.
- Have slurred speech.
- Cannot think well or clearly.
- Have slowed reflexes and breathing.
- Do not inject (“shoot-up”) Zubsolv.
- In an emergency, have family members tell the emergency department staff that you are physically dependent on an opioid and are being treated with Zubsolv.
- Never give anyone else your Zubsolv. They could die from taking it. Selling or giving away Zubsolv is against the law.
- Store Zubsolv securely, out of sight and reach of children, and in a location not accessible by others, including visitors to the home.
Who should not take Zubsolv?
Do not take Zubsolv if you are allergic to buprenorphine or naloxone.
What should I tell my healthcare provider before taking Zubsolv?
Zubsolv may not be right for you. Before taking Zubsolv, tell your doctor if you:
- Have trouble breathing or lung problems.
- Have an enlarged prostate gland (men).
- Have a head injury or brain problem.
- Have problems urinating.
- Have a curve in your spine that affects your breathing.
- Have liver or kidney problems.
- Have gallbladder problems.
- Have adrenal gland problems.
- Have Addison’s disease.
- Have low thyroid (hypothyroidism).
- Have a history of alcoholism.
- Have mental problems such as hallucinations (seeing or hearing things that are not there).
- Have any other medical condition.
- Are pregnant or plan to become pregnant. If you take Zubsolv while pregnant, your baby may have symptoms of opioid withdrawal or respiratory depression at birth. Talk to your doctor if you are pregnant or plan to become pregnant.
- Are breastfeeding or plan to breastfeed. Zubsolv can pass into your breast milk and may harm your baby. Talk to your doctor about the best way to feed your baby if you take Zubsolv. Monitor your baby for increased sleepiness and breathing problems.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Zubsolv may affect the way other medicines work and other medicines may affect how Zubsolv works. Some medicines may cause serious or life-threatening medical problems when taken with Zubsolv.
Sometimes the doses of certain medicines and Zubsolv may need to be changed if used together. Do not take any medicine while using Zubsolv until you have talked with your doctor. Your doctor will tell you if it is safe to take other medicines while you are using Zubsolv.
Be especially careful about taking other medicines that may make you sleepy, such as pain medicines, tranquilizers, sleeping pills, anxiety medicines, or antihistamines.
Know the medicines you take. Keep a list of them to show your doctor or pharmacist each time you get a new medicine.
How should I take Zubsolv?
- Always take Zubsolv exactly as your doctor tells you. Your doctor may change your dose after seeing how it affects you. Do not change your dose unless your doctor tells you to change it.
- Do not take Zubsolv more often than prescribed by your doctor.
- You may be prescribed a dose of 2 or more Zubsolv sublingual tablets at the same time.
- After induction (your first day of dosing), take Zubsolv 1 time a day.
- Do not cut, crush, break, chew or swallow the tablet. Your doctor should show you how to take Zubsolv the right way.
- Follow the same instructions every time you take a dose of Zubsolv.
- Zubsolv comes in a blister pack with 10 blister units. Each blister unit contains a Zubsolv tablet.
- Take the dose prescribed by your doctor as follows:
- Pull apart 1 of the blister units from the blister pack by tearing along the dotted lines (perforations) until it is fully separated.
- When the blister unit is fully separated, fold the single unit down at dotted line toward the blister.
- Slowly tear down at notch to open the blister unit.
- Do not push Zubsolv tablets through the foil. This could cause the tablet to break.
- As soon as you remove your prescribed dose of Zubsolv from the blister pack place the tablet under your tongue. If more than 1 tablet is required, place the tablets in different places under your tongue at the same time.
- Let the tablet dissolve completely. Zubsolv usually dissolves in your mouth within 5 minutes. If your mouth is dry, take a sip of water to moisten it. Spit out or swallow the water and dry your hands if they are wet before you place the Zubsolv tablet under your tongue.
- While Zubsolv is dissolving, do not chew or swallow the tablet because the medicine will not work as well.
- Do not eat or drink anything until the Zubsolv tablet has completely dissolved.
- Talking while the tablet is dissolving can affect how well the medicine in Zubsolv is absorbed.
- If you miss a dose of Zubsolv, take your medicine when you remember. If it is almost time for your next dose, skip the missed dose and take the next dose at your regular time. Do not take 2 doses at the same time unless your doctor tells you to. If you are not sure about your dosing, call your doctor.
- Do not stop taking Zubsolv suddenly. You could become sick and have withdrawal symptoms because your body has become used to the medicine. Physical dependence is not the same as drug addiction. Your doctor can tell you more about the differences between physical dependence and drug addiction. To have fewer withdrawal symptoms, ask your doctor how to stop using Zubsolv the right way.
- If you take too much Zubsolv go to the nearest hospital emergency room right away.
What should I avoid while taking Zubsolv?
- Do not drive, operate heavy machinery, or perform any other dangerous activities until you know how this medication affects you. Buprenorphine can cause drowsiness and slow reaction times. This may happen more often in the first few weeks of treatment when your dose is being changed, but can also happen if you drink alcohol or take other sedative drugs when you take Zubsolv.
You should not drink alcohol while taking Zubsolv, as this can lead to loss of consciousness or even death.
What are the possible side effects of Zubsolv?
Zubsolv can cause serious side effects, including:
- See “What is the most important information I should know about Zubsolv?”
- Respiratory problems. You have a higher risk of death and coma if you take Zubsolv with other medicines, such as benzodiazepines.
- Sleepiness, dizziness, and problems with coordination.
- Dependency or abuse.
- Liver problems. Call your doctor right away if you notice any of these signs of liver problems: Your skin or the white part of your eyes turning yellow (jaundice), urine turning dark, stools turning light in color, you have less of an appetite, or you have stomach (abdominal) pain or nausea. Your doctor should do tests before you start taking and while you take Zubsolv.
- Allergic reaction. You may have a rash, hives, swelling of your face, wheezing, or loss of blood pressure and consciousness. Call a doctor or get emergency help right away.
- Opioid withdrawal. This can include: shaking, sweating more than normal, feeling hot or cold more than normal, runny nose, watery eyes, goose bumps, diarrhea, vomiting and muscle aches. Tell your doctor if you develop any of these symptoms.
- Decrease in blood pressure. You may feel dizzy if you get up too fast from sitting or lying down.
The most common side effects of Zubsolv include:
- Headache
- Increased sweating
- Decrease in sleep (insomnia)
- Nausea
- Constipation
- Pain
- Vomiting
- Drug withdrawal syndrome
- Swelling of the extremities
Tell your doctor about any side effect that bothers you or that does not go away. These are not all the possible side effects of Zubsolv. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
General information about the safe and effective use of Zubsolv
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Zubsolv for a condition for which it was not prescribed. Do not give Zubsolv to other people, even if they have the same symptoms you have. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about Zubsolv. If you would like more information, talk to your doctor or pharmacist. You can ask your doctor or pharmacist for information that is written for health professionals.
For more information, call 1-888-ZUBSOLV (1-888-982-7658).
How should I store Zubsolv?
- Store Zubsolv at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Zubsolv in a safe place, out of the sight and reach of children.
How should I dispose of unused Zubsolv?
- Dispose of expired, unwanted, or unused Zubsolv by promptly flushing down the toilet, if a drug take-back option is not readily available. Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
What are the ingredients in Zubsolv?
Active Ingredients: buprenorphine and naloxone.
Inactive Ingredients: mannitol, citric acid, sodium citrate, microcrystalline cellulose, croscarmellose sodium, sucralose, silicon dioxide, sodium stearyl fumarate, and menthol flavor.
Label
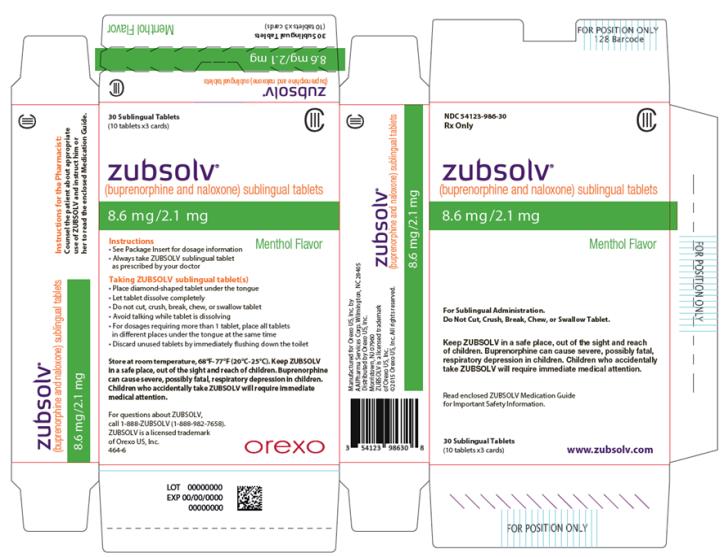
PRINCIPAL DISPLAY PANEL
- NDC 54123-986-30
Rx Only
CIII
zubsolv®
(buprenorphine and naloxone) sublingual tablets
8.6 mg/2.1 mg
Menthol Flavor
For Sublingual Administration.
Do Not Cut, Crush, Break, Chew, or Swallow Tablet.
Keep ZUBSOLV in a safe place, out of the sight and reach of children. Buprenorphine can cause severe, possibly fatal, respiratory depression in children. Children who accidentally take ZUBSOLV will require immediate medical attention.
Read enclosed ZUBSOLV Medication Guide for Important Safety Information.
30 Sublingual Tablets
(10 tablets x3 cards)
www.zubsolv.com
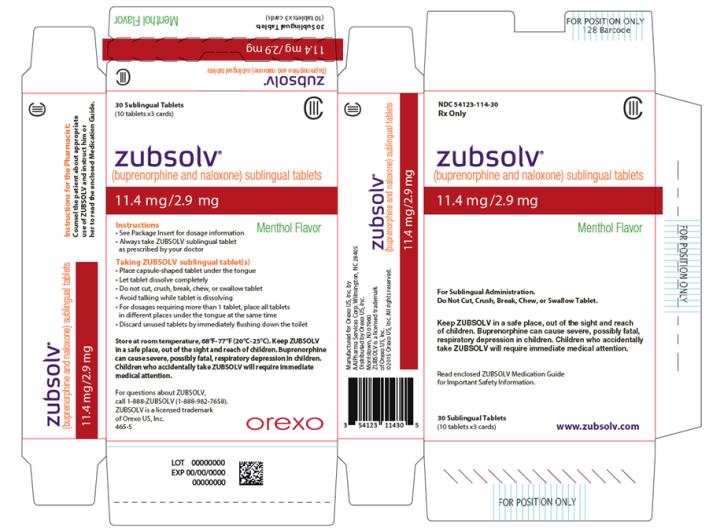
PRINCIPAL DISPLAY PANEL
- NDC 54123-114-30
Rx Only
CIII
zubsolv®
(buprenorphine and naloxone) sublingual tablets
11.4 mg/2.9 mg
Menthol Flavor
For Sublingual Administration.
Do Not Cut, Crush, Break, Chew, or Swallow Tablet.
Keep ZUBSOLV in a safe place, out of the sight and reach of children. Buprenorphine can cause severe, possibly fatal, respiratory depression in children. Children who accidentally take ZUBSOLV will require immediate medical attention.
Read enclosed ZUBSOLV Medication Guide for Important Safety Information.
30 Sublingual Tablets
(10 tablets x3 cards)
www.zubsolv.com
SRC: NLM .
