Xultophy
Generic name: insulin degludec and liraglutide
Medically reviewed by A Ras MD.
What is Xultophy?
Xultophy 100/3.6 is an injectable prescription medicine that contains 2 diabetes medicines, insulin degludec, 100 units/mL, and liraglutide, 3.6 mg/mL. Xultophy 100/3.6 should be used along with diet and exercise to lower blood sugar (glucose) in adults with type 2 diabetes mellitus. Xultophy 100/3.6 is not recommended as the first choice of medicine for treating diabetes.
Xultophy 100/3.6 is not recommended for use in combination with any other product containing liraglutide or another glucagon-like peptide 1 receptor agonist (GLP-1 receptor agonist). Xultophy 100/3.6 is not for use in people with type 1 diabetes or people with diabetic ketoacidosis (increased ketones in the blood or urine). It is not known if Xultophy 100/3.6 can be used with mealtime insulin.
It is not known if Xultophy 100/3.6 is safe and effective for use in children.
Description
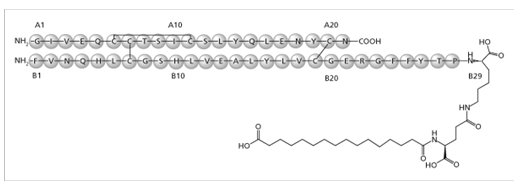
Insulin degludec
Insulin degludec is a long-acting basal human insulin analog. Insulin degludec is produced by a process that includes expression of recombinant DNA in Saccharomyces cerevisiae followed by chemical modification.
Insulin degludec differs from human insulin in that the amino acid threonine in position B30 has been omitted and a side-chain consisting of glutamic acid and a C16 fatty acid has been attached (chemical name: LysB29(Nε-hexadecandioyl-γ-Glu) des(B30) human insulin). Insulin degludec has a molecular formula of C274H411N65O81S6 and a molecular weight of 6.104 kDa. It has the following structure:
Figure 1: Structural Formula of Insulin degludec
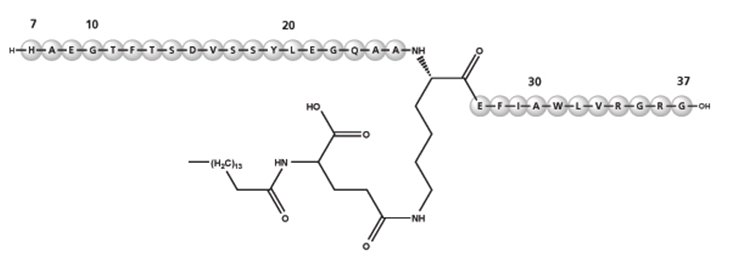
Liraglutide
Liraglutide is an analog of human GLP-1 and acts as a GLP-1 receptor agonist. The peptide precursor of liraglutide, produced by a process that includes expression of recombinant DNA in Saccharomyces cerevisiae, has been engineered to be 97% homologous to native human GLP-1 by substituting arginine for lysine at position 34. Liraglutide is made by attaching a C16 fatty acid (palmitic acid) with a glutamic acid spacer on the remaining lysine residue at position 26 of the peptide precursor. The molecular formula of liraglutide is C172H265N43O51 and the molecular weight is 3.751 kDa. It has the following structure:
Figure 2: Structural Formula of Liraglutide
XULTOPHY 100/3.6 (insulin degludec and liraglutide) injection, for subcutaneous use, is a combination of a long-acting basal human insulin analog, insulin degludec, and a GLP-1 receptor agonist, liraglutide.
XULTOPHY 100/3.6 is a sterile, aqueous, clear, and colorless solution. Each pre-filled pen contains 3 mL equivalent to 300 units insulin degludec and 10.8 mg liraglutide. Each mL contains 100 units insulin degludec and 3.6 mg liraglutide.
XULTOPHY 100/3.6 contains the following inactive ingredients per mL: glycerol (19.7 mg), phenol (5.7 mg), zinc (55 mcg), and Water for Injection, USP. XULTOPHY 100/3.6 has a pH of approximately 8.15. Hydrochloric acid or sodium hydroxide may be added to adjust pH.
Mechanism of Action
XULTOPHY 100/3.6
XULTOPHY 100/3.6 is a combination product consisting of insulin degludec and liraglutide.
Insulin degludec
The primary activity of insulin degludec is the regulation of glucose metabolism. Insulin and its analogs lower blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin also inhibits lipolysis and proteolysis, and enhances protein synthesis.
Liraglutide
Liraglutide is a Glucagon-Like Peptide-1 (GLP-1) receptor agonist that increases glucose-dependent insulin release, decreases glucagon secretion, and slows gastric emptying.
What is the most important information I should know about Xultophy?
Xultophy 100/3.6 may cause serious side effects, including:
- Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rats and mice, liraglutide, one of the components of Xultophy 100/3.6, and medicines that work like liraglutide caused thyroid tumors, including thyroid cancer. It is not known if Xultophy 100/3.6 will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
- Do not use Xultophy 100/3.6 if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC), or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Who should not take Xultophy?
Do not use Xultophy 100/3.6 if:
- you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- you are allergic to insulin degludec, liraglutide or any of the ingredients in Xultophy 100/3.6. See the end of this Medication Guide for a complete list of ingredients in Xultophy 100/3.6.
- you are having an episode of low blood sugar (hypoglycemia).
What should I tell my healthcare provider before taking Xultophy?
Before using Xultophy 100/3.6, tell your healthcare provider about all your medical conditions, including if you:
- have or have had problems with your pancreas, kidneys, or liver.
- have heart failure or other heart problems. If you have heart failure, it may get worse while you take TZDs with Xultophy 100/3.6.
- have severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food.
- are taking certain medicines called GLP-1 receptor agonists.
- have had an allergic reaction to a GLP-1 receptor agonist medicine.
- are pregnant or plan to become pregnant. It is not known if Xultophy 100/3.6 will harm your unborn baby. Tell your healthcare provider if you become pregnant or think you may be pregnant while using Xultophy 100/3.6.
- are breastfeeding or plan to breastfeed. It is not known if Xultophy 100/3.6 passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while using Xultophy 100/3.6.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Xultophy 100/3.6 may affect the way some medicines work, and some medicines may affect the way Xultophy 100/3.6 works. Before using Xultophy 100/3.6, talk to your healthcare provider about low blood sugar and how to manage it. Tell your healthcare provider if you are taking other medicines to treat diabetes.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Xultophy?
- Read the Instructions for Use that comes with Xultophy 100/3.6.
- Use Xultophy 100/3.6 exactly as your healthcare provider tells you to.
- Do not change your dosing schedule without first talking to your healthcare provider. The dose counter on your Xultophy 100/3.6 pen shows the number of units of Xultophy 100/3.6 to be injected.
- Your healthcare provider should show you how to use Xultophy 100/3.6 before you use it for the first time.
- Xultophy 100/3.6 is injected under the skin (subcutaneously) of your thigh, upper arm or stomach (abdomen).
- Do not inject Xultophy 100/3.6 into a muscle (intramuscularly) or vein (intravenously) or use in an insulin infusion pump.
- Use Xultophy 100/3.6 at the same time each day with or without food.
- If you miss a dose of Xultophy 100/3.6, resume your 1 time daily dosing schedule at the next scheduled dose. Do not take 2 doses at the same time or increase your dose to make up for the missed dose. If you miss more than 3 days of Xultophy 100/3.6, call your healthcare provider for further instructions about taking Xultophy 100/3.6 at the right dose and to help lower your chance of having an upset stomach.
- Do not dilute Xultophy 100/3.6 with any other liquids.
- Do not mix Xultophy 100/3.6 with any other insulin products or GLP-1 receptor agonists in the same injection.
- Do not split your dose of Xultophy 100/3.6. Give your full dose of Xultophy 100/3.6 in 1 injection.
- Check the Pen label each time you give your injection to make sure you are using the correct medicine.
- Do not take more than 50 units of Xultophy 100/3.6 each day. Xultophy 100/3.6 contains two medicines: insulin degludec and liraglutide. If you take too much Xultophy 100/3.6, it can cause severe nausea and vomiting. Do not take Xultophy 100/3.6 with other GLP-1 receptor agonists. If you take too much Xultophy 100/3.6, call your healthcare provider or go to the nearest hospital emergency room right away.
- Change (rotate) your injection sites within the area you choose with each injection to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not use the exact same spot for each injection.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Do not share your Xultophy 100/3.6 pen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
Check your blood sugar levels. Ask your healthcare provider what your blood sugars should be and when you should check your blood sugar levels.
Your dose of Xultophy 100/3.6 and other diabetes medicines may need to change because of:
- change in level of physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, or because of other medicines you take.
What should I avoid while taking Xultophy?
While taking Xultophy 100/3.6 do not:
- drive or operate heavy machinery, until you know how Xultophy 100/3.6 affects you.
- drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of Xultophy?
Xultophy 100/3.6 may cause serious side effects that can lead to death, including:
- See “What is the most important information I should know about Xultophy 100/3.6?”
- inflammation of your pancreas (pancreatitis). Stop using Xultophy 100/3.6 and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
- low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Xultophy 100/3.6 with another medicine that can cause low blood sugar.Signs and symptoms of low blood sugar may include:
- dizziness or light-headedness
- sweating
- confusion or drowsiness
- headache
- blurred vision
- slurred speech
- shakiness
- fast heartbeat
- anxiety, irritability, or mood changes
- hunger
- weakness
- feeling jittery
- kidney problems (kidney failure). Worsening of kidney failure and sudden kidney failure have happened in people with kidney problems and in people without kidney problems, who have taken liraglutide, one of the ingredients in Xultophy 100/3.6.Diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems to get worse. Tell your healthcare provider if you have diarrhea, nausea, or vomiting. Drink plenty of fluids to help reduce your risk of dehydration during treatment with Xultophy 100/3.6.
- serious allergic reactions. Stop using Xultophy 100/3.6 and get medical help right away, if you have any symptoms of a serious allergic reaction including:
- hives
- rash
- itching
- fast heartbeat
- fainting or feeling dizzy
- swelling of your face, lips, tongue, or throat
- problems breathing or swallowing
- sudden coughing
- chest pain or tightness
- gallbladder problems. Gallbladder problems have happened in some people who take liraglutide, an ingredient in Xultophy 100/3.6. Tell your healthcare provider right away if you get symptoms of gallbladder problems which may include:
- pain in the right or middle upper stomach area
- fever
- nausea and vomiting
- your skin or the white part of your eyes turns yellow
- low potassium in your blood (hypokalemia).
- heart failure. Taking certain diabetes pills called thiazolidinediones or TZDs with Xultophy 100/3.6 may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure, it may get worse while you take TZDs with Xultophy 100/3.6. Your healthcare provider should monitor you closely while you are taking TZDs with Xultophy 100/3.6. Tell your healthcare provider if you have any new or worse symptoms of heart failure including shortness of breath, tiredness, swelling of your ankles or feet and sudden weight gain. Treatment with TZDs and Xultophy 100/3.6 may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
The most common side effects of Xultophy 100/3.6 include stuffy or runny nose, sore throat, headache, nausea, diarrhea, increased blood levels of lipase, and upper respiratory tract infection. Talk to your healthcare provider about any side effect that bothers you or does not go away.
These are not all the possible side effects of Xultophy 100/3.6.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Keep Xultophy 100/3.6 and all medicines out of the reach of children.
General information about the safe and effective use of Xultophy
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Xultophy 100/3.6 for a condition for which it was not prescribed. Do not give Xultophy 100/3.6 to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about Xultophy 100/3.6 that is written for health professionals.
How should I store Xultophy?
Prior to first use, Xultophy 100/3.6 should be stored between 2°C and 8°C (36°F to 46°F) until the expiration date printed on the label. Store prefilled pens in the carton so they will stay clean and protected from light. Do not store in the freezer or directly adjacent to the refrigerator cooling element. Do not freeze. Do not use Xultophy 100/3.6 if it has been frozen.
After first use, the Xultophy 100/3.6 pen can be stored for 21 days at controlled room temperature (59°F to 86°F; 15°C to 30°C) or in a refrigerator (36°F to 46°F; 2°C to 8°C). Keep all Xultophy 100/3.6 pens away from direct heat and light.
Always remove the needle after each injection and store the Xultophy 100/3.6 pen without a needle attached. This prevents contamination and/or infection, or leakage of the Xultophy 100/3.6 pen, and will ensure accurate dosing. Always use a new needle for each injection to prevent contamination.
Storage Conditions for Xultophy 100/3.6 Pen
| Prior to first use | After first use | After first use |
| Refrigerated 36°F to 46°F (2°C to 8°C) |
Room Temperature 59°F to 86°F (15°C to 30°C) |
Refrigerated 36°F to 46°F (2°C to 8°C) |
| Until expiration date | 21 Days | 21 Days |
What are the ingredients in Xultophy?
Active Ingredients: insulin degludec and liraglutide
Inactive Ingredients: glycerol, phenol, zinc, and water for injection. Hydrochloric acid or sodium hydroxide may be added to adjust pH.
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- Xultophy®100/3.6
- NDC 0169-2911-15
- List: 291115
- (insulin degludec and liraglutide) injection
- For Single Patient Use Only
- 100 units/mL and 3.6 mg/mL
- With each unit of insulin degludec, the pen
- also delivers 0.036 mg of liraglutide
- 5×3 mL Prefilled Pens
- For subcutaneous use only
- Recommended for use with NovoFine®,
- Novofine® Plus or NovoTwist® disposable needles.
- Must be refrigerated until first use.
- Store at 36°F – 46°F (2°C – 8°C).
- Do not freeze.
- Protect from light.
- Rx Only
- Dispense in this sealed carton.
- ATTENTION: Dispense the enclosed Medication Guide to each patient.
SRC: NLM .
