Xigduo XR
Generic name: dapagliflozin and metformin
Drug class: Antidiabetic combinations
Medically reviewed by A Ras MD.
What is Xigduo XR?
Xigduo XR contains 2 prescription medicines called dapagliflozin (Farxiga) and metformin HCl (Glucophage). Xigduo XR is used to improve blood sugar (glucose) control along with diet and exercise in adults with type 2 diabetes reduce the risk of hospitalization for heart failure in adults with type 2 diabetes and known cardiovascular disease or multiple cardiovascular risk factors
Xigduo XR is not for people with type 1 diabetes. Xigduo XR is not for people with diabetic ketoacidosis (increased ketones in your blood or urine).
It is not known if Xigduo XR is safe and effective in children younger than 18 years of age.
Description
XIGDUO XR tablets contain: dapagliflozin, a SGLT2 inhibitor, and metformin HCl, a biguanide.
Dapagliflozin
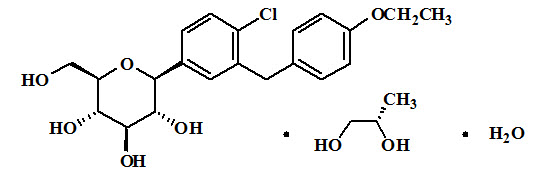
Dapagliflozin is described chemically as D-glucitol, 1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-, (1S)-, compounded with (2S)-1,2-propanediol, hydrate (1:1:1). The empirical formula is C21H25ClO6•C3H8O2•H2O and the formula weight is 502.98. The structural formula is:
Metformin hydrochloride
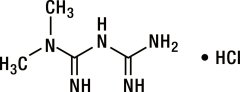
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is a white to off-white crystalline compound with a molecular formula of C4H11N5•HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water, slightly soluble in alcohol, and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is:
XIGDUO XR
XIGDUO XR is available for oral administration as tablets containing the equivalent of 2.5 mg dapagliflozin as dapagliflozin propanediol and 1,000 mg metformin hydrochloride which is equivalent to 779.86 mg metformin base (XIGDUO XR 2.5 mg/1,000 mg), 5 mg dapagliflozin as dapagliflozin propanediol and 500 mg metformin hydrochloride which is equivalent to 389.9 mg metformin base (XIGDUO XR 5 mg/500 mg), the equivalent of 5 mg dapagliflozin as dapagliflozin propanediol and 1,000 mg metformin hydrochloride which is equivalent to 779.86 mg metformin base (XIGDUO XR 5 mg/1,000 mg), the equivalent of 10 mg dapagliflozin as dapagliflozin propanediol and 500 mg metformin hydrochloride which is equivalent to 389.9 mg metformin base (XIGDUO XR 10 mg/500 mg), or the equivalent of 10 mg dapagliflozin as dapagliflozin propanediol and 1,000 mg metformin hydrochloride which is equivalent to 779.86 mg metformin base (XIGDUO XR 10 mg/1,000 mg).
Each film-coated tablet of XIGDUO XR contains the following inactive ingredients: microcrystalline cellulose, lactose anhydrous, crospovidone, silicon dioxide, magnesium stearate, carboxymethylcellulose sodium, and hypromellose.
The film coatings contain the following inactive ingredients: polyvinyl alcohol, titanium dioxide, polyethylene glycol, and talc. Additionally, the film coating for the XIGDUO XR 5 mg/500 mg tablets contains FD&C Yellow No. 6/Sunset Yellow FCF aluminum lake. The film coating for the XIGDUO XR 2.5 mg/1,000 mg, 5 mg/1,000 mg, 10 mg/500 mg, and 10 mg/1,000 mg tablets contains iron oxides.
Mechanism of Action
Dapagliflozin
Sodium-glucose cotransporter 2 (SGLT2), expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen. Dapagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose, and thereby promotes urinary glucose excretion. Dapagliflozin also reduces sodium reabsorption and increases the delivery of sodium to the distal tubule. This may influence several physiological functions including, but not restricted to, lowering both pre- and afterload of the heart and downregulation of sympathetic activity, and decreased intraglomerular pressure which is believed to be mediated by increased tubuloglomerular feedback.
Metformin HCl
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may decrease.
What is the most important information I should know about Xigduo XR?
Xigduo XR can cause serious side effects, including:
- Lactic Acidosis. Metformin, one of the medicines in Xigduo XR, can cause a rare but serious condition called lactic acidosis (a build-up of an acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in the hospital.Call your healthcare provider right away if you have any of the following symptoms, which could be signs of lactic acidosis:
Most people who have had lactic acidosis with metformin have other things that, combined with the metformin use, led to the lactic acidosis. Tell your healthcare provider if you have any of the following, because you have a higher chance for getting lactic acidosis with Xigduo XR if you:
- have severe kidney problems or your kidneys are affected by certain x-ray tests that use injectable dye.
- have liver problems.
- drink alcohol very often, or drink a lot of alcohol in short-term “binge” drinking.
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
- have surgery.
- have a heart attack, severe infection, or stroke.
The best way to keep from having a problem with lactic acidosis from metformin is to tell your healthcare provider if you have any of the problems in the list above. Your healthcare provider may decide to stop your Xigduo XR for a while if you have any of these things.
Xigduo XR can have other serious side effects. See “What are the possible side effects of Xigduo XR?”
Who should not take Xigduo XR?
Do not take Xigduo XR if you:
- have moderate to severe kidney problems or are on dialysis.
- are allergic to dapagliflozin, metformin HCl, or any of the ingredients in Xigduo XR. See the end of this Medication Guide for a list of ingredients in Xigduo XR. Symptoms of a serious allergic reaction to Xigduo XR may include:
- skin rash
- raised red patches on your skin (hives)
- swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing
- If you have any of these symptoms, stop taking Xigduo XR and contact your healthcare provider or go to the nearest hospital emergency room right away.
- have a condition called metabolic acidosis or diabetic ketoacidosis (increased ketones in your blood or urine).
What should I tell my healthcare provider before taking Xigduo XR?
Before you take Xigduo XR, tell your healthcare provider if you:
- have type 1 diabetes or have had diabetic ketoacidosis.
- have kidney problems.
- have liver problems.
- have a history of urinary tract infections or problems urinating.
- have heart problems, including congestive heart failure.
- are going to have surgery and will not be able to eat or drink much. Your doctor may stop Xigduo XR before you have surgery. Talk to your doctor if you are having surgery about when to stop taking Xigduo XR and when to start it again. See “What is the most important information I should know about Xigduo XR?”
- are eating less, or there is a change in your diet.
- have or have had problems with your pancreas, including pancreatitis or surgery on your pancreas.
- drink alcohol very often, or drink a lot of alcohol in the short-term (“binge” drinking).
- are going to get an injection of dye or contrast agents for an x-ray procedure. Xigduo XR may need to be stopped for a short time. Talk to your healthcare provider about when you should stop Xigduo XR and when you should start Xigduo XR again. See “What is the most important information I should know about Xigduo XR?”
- are pregnant or plan to become pregnant. Xigduo XR may harm your unborn baby. If you are pregnant or plan to become pregnant, talk to your healthcare provider about the best way to control your blood sugar.
- are breastfeeding or plan to breastfeed. It is not known if Xigduo XR passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby if you are taking Xigduo XR.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Xigduo XR may affect the way other medicines work and other medicines may affect the way Xigduo XR works. Especially tell your healthcare provider if you take:
- water pills (diuretics)
- rifampin (used to treat or prevent tuberculosis)
- phenytoin (used to control seizures)
- digoxin (used to treat heart problems)
Ask your healthcare provider for a list of these medicines if you are not sure if your medicine is listed above.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take Xigduo XR?
- Take Xigduo XR exactly as your healthcare provider tells you to take it.
- Do not change your dose of Xigduo XR without talking to your healthcare provider.
- Take Xigduo XR by mouth 1 time each day with meals to lower your chance of an upset stomach. Talk to your healthcare provider about the best time of day for you.
- Swallow Xigduo XR whole. Do not crush, cut, or chew Xigduo XR.
- You may sometimes pass a soft mass in your stools (bowel movement) that looks like Xigduo XR tablets.
- When your body is under some types of stress, such as fever, trauma (such as a car accident), infection, or surgery, the amount of diabetes medicine you need may change. Tell your healthcare provider right away if you have any of these conditions and follow your healthcare provider’s instructions.
- Stay on your prescribed diet and exercise program while taking Xigduo XR.
- Xigduo XR will cause your urine to test positive for glucose.
- Your healthcare provider may do certain blood tests before you start Xigduo XR and during your treatment.
- Your healthcare provider will check your diabetes with regular blood tests, including your blood sugar levels and your A1c.
- Follow your healthcare provider’s instructions for treating low blood sugar (hypoglycemia). Talk to your healthcare provider if low blood sugar is a problem for you.
- If you miss a dose of Xigduo XR, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose and take the medicine at the next regularly scheduled time.
- If you take too much Xigduo XR, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking Xigduo XR?
Avoid drinking alcohol very often, or drinking a lot of alcohol in a short period of time (“binge” drinking). It can increase your chances of getting serious side effects.
What are the possible side effects of Xigduo XR?
Xigduo XR may cause serious side effects including:
See “What is the most important information I should know about Xigduo XR?”
- Dehydration. Xigduo XR can cause some people to become dehydrated (the loss of body water and salt). Dehydration may cause you to feel dizzy, faint, lightheaded, or weak, especially when you stand up (orthostatic hypotension).
You may be at a higher risk of dehydration if you:- have low blood pressure
- take medicines to lower your blood pressure, including water pills (diuretics)
- are 65 years of age or older
- are on a low salt diet
- have kidney problems
Talk to your healthcare provider about what you can do to prevent dehydration including how much fluid you should drink on a daily basis.
- Ketoacidosis (increased ketones in your blood or urine). Ketoacidosis has happened in people who have type 1 diabetes or type 2 diabetes, during treatment with dapagliflozin, one of the medicines in Xigduo XR. Ketoacidosis has also happened in people with diabetes who were sick or who had surgery during treatment with Xigduo XR. Ketoacidosis is a serious condition, which may need to be treated in a hospital. Ketoacidosis may lead to death. Ketoacidosis can happen with Xigduo XR even if your blood sugar is less than 250 mg/dL. Stop taking Xigduo XR and call your healthcare provider right away if you get any of the following symptoms:
- nausea
- vomiting
- stomach area (abdominal) pain
- tiredness
- trouble breathing
If you get any of these symptoms during treatment with Xigduo XR, if possible check for ketones in your urine, even if your blood sugar is less than 250 mg/dL.
- Kidney problems. Sudden kidney injury has happened to people taking Xigduo XR. Call your healthcare provider right away if you:
- reduce the amount of food or liquid you drink, for example, if you are sick and cannot eat or
- you start to lose liquids from your body, for example, from vomiting, diarrhea, or being in the sun too long.
- Serious urinary tract infections. Serious urinary tract infections that may lead to hospitalization have happened in people who are taking dapagliflozin, one of the medicines in Xigduo XR. Tell your healthcare provider if you have any signs or symptoms of a urinary tract infection, such as a burning feeling when passing urine, a need to urinate often, the need to urinate right away, pain in the lower part of your stomach (pelvis), or blood in the urine. Sometimes people also may have a fever, back pain, nausea or vomiting.
- Low blood sugar (hypoglycemia). If you take Xigduo XR with another medicine that can cause low blood sugar, such as sulfonylureas or insulin, your risk of getting low blood sugar is higher. The dose of your sulfonylurea medicine or insulin may need to be lowered while you take Xigduo XR. Signs and symptoms of low blood sugar may include:
- headache
- shaking or feeling jittery
- dizziness
- weakness
- sweating
- fast heartbeat
- confusion
- drowsiness
- irritability
- hunger
- A rare but serious bacterial infection that causes damage to the tissue under the skin (necrotizing fasciitis) in the area between and around the anus and genitals (perineum). Necrotizing fasciitis of the perineum has happened in women and men who take dapagliflozin, one of the medicines in Xigduo XR. Necrotizing fasciitis of the perineum may lead to hospitalization, may require multiple surgeries and may lead to death. Seek medical attention immediately if you have fever or you are feeling very weak, tired or uncomfortable (malaise) and you develop any of the following symptoms in the area between and around the anus and genitals:
- pain or tenderness
- swelling
- redness of skin (erythema)
- Low vitamin B12 (vitamin B12 deficiency). Using metformin for long periods of time may cause a decrease in the amount of vitamin B12 in your blood, especially if you have had low vitamin B12 levels before. Your healthcare provider may do blood tests to check your vitamin B12 levels.
- Vaginal yeast infection. Women who take Xigduo XR may get vaginal yeast infections. Symptoms of a vaginal yeast infection include:
- vaginal odor
- white or yellowish vaginal discharge (discharge may be lumpy or look like cottage cheese)
- vaginal itching
- Yeast infection of the penis (balanitis). Men who take Xigduo XR may get a yeast infection of the skin around the penis. Certain men who are not circumcised may have swelling of the penis that makes it difficult to pull back the skin around the tip of the penis. Other symptoms of yeast infection of the penis include:
- redness, itching, or swelling of the penis
- rash of the penis
- foul smelling discharge from the penis
- pain in the skin around the penis
Talk to your healthcare provider about what to do if you get symptoms of a yeast infection of the vagina or penis. Your healthcare provider may suggest you use an over-the-counter antifungal medicine. Talk to your healthcare provider right away if you use an over-the-counter antifungal medication and your symptoms do not go away.
The most common side effects of Xigduo XR include:
- vaginal yeast infections and yeast infections of the penis
- diarrhea
- headache
- stuffy or runny nose and sore throat
- urinary tract infection
Tell your healthcare provider or pharmacist if you have any side effect that bothers you or does not go away.
These are not all of the possible side effects of Xigduo XR. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Xigduo XR
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Xigduo XR for a condition for which it is not prescribed. Do not give Xigduo XR to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about Xigduo XR. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about Xigduo XR that is written for healthcare professionals.
For more information, go to www.xigduoxr.com or call 1-800-236-9933.
How should I store Xigduo XR?
Store Xigduo XR at room temperature between 68°F and 77°F (20°C and 25°C).
Keep Xigduo XR and all medicines out of the reach of children.
What are the ingredients in Xigduo XR?
Active ingredients: dapagliflozin and metformin hydrochloride
Inactive ingredients: microcrystalline cellulose, lactose anhydrous, crospovidone, silicon dioxide, magnesium stearate, carboxymethylcellulose sodium, and hypromellose.
The film coatings contain the following inactive ingredients: polyvinyl alcohol, titanium dioxide, polyethylene glycol, and talc. Additionally, the film coating for the Xigduo XR 5 mg/500 mg tablets contains FD&C Yellow No. 6/Sunset Yellow FCF Aluminum Lake and the film coating for the Xigduo XR 2.5 mg/1000 mg, 5 mg/1000 mg, 10 mg/500 mg, and 10 mg/1000 mg tablets contains iron oxides.
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 2.5 MG/1000 MG
- 60 Tablets NDC 0310-6225-60
- xigduo® XR
- (dapagliflozin/metformin HCl
- extended-release) tablets
- 2.5 mg/1000 mg
- Dispense with Medication Guide
- Rx only
- Do not crush, cut, or chew tablets.
- Tablets must be swallowed whole.
- AstraZeneca

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 5 MG/500 MG
- 30 Tablets NDC 0310-6250-30
- xigduo® XR
- (dapagliflozin/metformin HCl
- extended-release) tablets
- 5 mg/500 mg
- Dispense with Medication Guide
- Rx only
- Do not crush, cut, or chew tablets.
- Tablets must be swallowed whole.
- AstraZeneca

SRC: NLM .
