Welireg
Generic name: belzutifan
Dosage form: tablets
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Welireg?
Welireg is a prescription medicine used to treat adults with von Hippel-Lindau (VHL) disease who need treatment for a type of kidney cancer called renal cell carcinoma (RCC), tumors in the brain and spinal cord called central nervous system hemangioblastomas, or a type of pancreatic cancer called pancreatic neuroendocrine tumors, that do not require surgery right away.
It is not known if Welireg is safe and effective in children.
Description
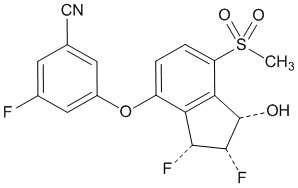
Belzutifan is an inhibitor of hypoxia-inducible factor-2α (HIF-2α). The chemical name of belzutifan is 3-[[(1S,2S,3R)-2,3-Difluoro-2,3-dihydro-1-hydroxy-7-(methylsulfonyl)-1H-inden-4-yl]oxy]-5-fluorobenzonitrile. The molecular formula is C17H12F3NO4S and the molecular weight is 383.34 Daltons. The chemical structure is:
 |
Belzutifan is a white to light brown powder that is soluble in acetonitrile, dimethoxyethane, and acetone, sparingly soluble in ethyl acetate, very slightly soluble in isopropanol and toluene, and insoluble in water.
WELIREG is supplied as blue, film-coated tablets for oral use containing 40 mg of belzutifan together with croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, mannitol, microcrystalline cellulose, and silicon dioxide, as inactive ingredients. In addition, the film-coating contains FD&C Blue #2 aluminum lake, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide.
Mechanism of Action
Belzutifan is an inhibitor of hypoxia-inducible factor 2 alpha (HIF-2α). HIF-2α is a transcription factor that plays a role in oxygen sensing by regulating genes that promote adaptation to hypoxia. Under normal oxygen levels, HIF-2α is targeted for ubiquitin-proteasomal degradation by VHL protein. Lack of functional VHL protein results in stabilization and accumulation of HIF-2α. Upon stabilization, HIF-2α translocates into the nucleus and interacts with hypoxia-inducible factor 1 beta (HIF-1β) to form a transcriptional complex that induces expression of downstream genes, including genes associated with cellular proliferation, angiogenesis, and tumor growth. Belzutifan binds to HIF-2α, and in conditions of hypoxia or impairment of VHL protein function, belzutifan blocks the HIF-2α-HIF-1β interaction, leading to reduced transcription and expression of HIF-2α target genes. In vivo, belzutifan demonstrated anti-tumor activity in mouse xenograft models of renal cell carcinoma.
What is the most important information I should know about Welireg?
Welireg may cause serious side effects, including:
- Low red blood cell counts (anemia). Low red blood cell counts are common with Welireg and can be severe. You may need a blood transfusion if your red blood cell counts drop too low. Your healthcare provider will do blood tests to check your red blood cell counts before you start and during treatment with Welireg. Tell your healthcare provider if you get any symptoms of low red blood cell counts, including tiredness, feeling cold, shortness of breath, chest pain, or fast heartbeat.
- Low oxygen levels in your body. Welireg can cause low oxygen levels in your body that can be severe and may require you to stop treatment with Welireg, receive oxygen therapy, or be hospitalized. Your healthcare provider will monitor your oxygen levels before you start and during treatment with Welireg. Tell your healthcare provider or get medical help right away if you get symptoms of low oxygen in your body, including shortness of breath or increased heart rate.
- Harm to your unborn baby. Treatment with Welireg during pregnancy can cause harm to your unborn baby.
- Females who are able to become pregnant:
- Your healthcare provider will do a pregnancy test before you start treatment with Welireg.
- You should use an effective form of non-hormonal birth control (contraception) during treatment with Welireg and for 1 week after your last dose.
- Birth control methods that contain hormones (such as birth control pills, injections, or transdermal system patches) may not work as well during treatment with Welireg.
- Talk to your healthcare provider about birth control methods that may be right for you during treatment with Welireg.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Welireg.
- Males with female partners who are able to become pregnant:
- You should use effective birth control (contraception) during treatment with Welireg and for 1 week after your last dose.
- Tell your healthcare provider right away if your partner becomes pregnant or thinks she is pregnant while you are taking Welireg.
- Females who are able to become pregnant:
See “What are the possible side effects of Welireg?” for more information about side effects.
What should I tell my healthcare provider before taking Welireg?
Before taking Welireg, tell your healthcare provider about all of your medical conditions, including if you:
- have low red blood cell counts (anemia)
- are pregnant or plan to become pregnant. See “What is the most important information I should know about Welireg?”
- are breastfeeding or plan to breastfeed. It is not known if Welireg passes into your breast milk. Do not breastfeed during treatment with Welireg and for 1 week after your last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Welireg and certain other medicines can affect each other and cause serious side effects.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take Welireg?
- Take Welireg exactly as your healthcare provider tells you.
- Do not stop taking Welireg or change your dose without talking to your healthcare provider.
- Take your prescribed dose of Welireg 1 time a day, at the same time each day.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Welireg if you have certain side effects.
- Take Welireg with or without food.
- Swallow Welireg tablets whole. Do not chew, crush, or split Welireg tablets.
- If you miss a dose of Welireg, take it as soon as possible on the same day. Then take your next dose of Welireg at your regular time the next day. Do not take extra tablets to make up for the missed dose.
- If you vomit after taking a dose of Welireg, do not take an extra dose. Take your next dose at your regular time the next day.
- If you take too much Welireg, call your healthcare provider or go to the nearest emergency room right away.
What are the possible side effects of Welireg?
Welireg may cause serious side effects, including:
- See “What is the most important information I should know about Welireg?”
The most common side effects of Welireg include:
- feeling tired
- increased creatinine (kidney function test)
- headache
- feeling dizzy
- increased blood sugar (glucose) levels
- nausea
Welireg may cause fertility problems in males and females, which may affect your ability to have children. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of Welireg.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Welireg
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Welireg for a condition for which it was not prescribed. Do not give Welireg to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Welireg that is written for health professionals.
How should I store Welireg?
- Store Welireg at room temperature between 68°F to 77°F (20°C to 25°C).
- The Welireg bottle contains 2 desiccant canisters that help keep your medicine dry. Do not eat the desiccant canisters.
Keep Welireg and all medicines out of the reach of children.
What are the ingredients in Welireg?
Active ingredient: belzutifan
Inactive ingredients: croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, mannitol, microcrystalline cellulose, and silicon dioxide. The film-coating contains FD&C Blue #2 aluminum lake, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Label
PRINCIPAL DISPLAY PANEL – 40 MG BOTTLE LABEL
- NDC 0006-5331-01
- WeliregTM
(belzutifan) tablets - 40 mg
- Dispense the accompanying Medication
Guide to each patient. - Each tablet contains 40 mg of belzutifan.
- Rx only
- 90 Tablets
![]()
SRC: NLM .
