Vyvanse
Generic name: lisdexamfetamine
Drug class: CNS stimulants
Medically reviewed by A Ras MD.
What is Vyvanse?
Vyvanse is a central nervous system stimulant prescription medicine used to treat Attention-Deficit/Hyperactivity Disorder (ADHD). Vyvanse may help increase attention and decrease impulsiveness and hyperactivity in patients with ADHD.
It is also used in Binge Eating Disorder (BED). Vyvanse may help reduce the number of binge eating days in patients with BED.
Vyvanse is not for weight loss. It is not known if Vyvanse is safe and effective for the treatment of obesity.
It is not known if Vyvanse is safe and effective in children with ADHD under 6 years of age or in patients with BED under 18 years of age.
Description
VYVANSE (lisdexamfetamine dimesylate), a CNS stimulant, is for once-a-day oral administration. The chemical designation for lisdexamfetamine dimesylate is (2S)-2,6-diamino-N-[(1S)-1-methyl-2-phenylethyl] hexanamide dimethanesulfonate. The molecular formula is C15H25N3O∙(CH4O3S)2, which corresponds to a molecular weight of 455.60. The chemical structure is:
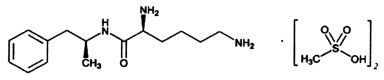
Lisdexamfetamine dimesylate is a white to off-white powder that is soluble in water (792 mg/mL).
Information for VYVANSE capsules:
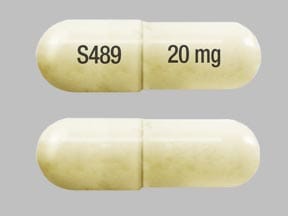
VYVANSE capsules contain 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, and 70 mg of lisdexamfetamine dimesylate (equivalent to 5.8 mg, 11.6 mg, 17.3 mg, 23.1 mg, 28.9 mg, 34.7 mg, and 40.5 mg of lisdexamfetamine).
Inactive ingredients: microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. The capsule shells contain gelatin, titanium dioxide, and one or more of the following: FD&C Red #3, FD&C Yellow #6, FD&C Blue #1, Black Iron Oxide, and Yellow Iron Oxide.
Information for VYVANSE chewable tablets:
VYVANSE chewable tablets contain 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, and 60 mg of lisdexamfetamine dimesylate (equivalent to 5.8 mg, 11.6 mg, 17.3 mg, 23.1 mg, 28.9 mg, and 34.7 mg of lisdexamfetamine).
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, guar gum, magnesium stearate, mannitol, microcrystalline cellulose, sucralose, artificial strawberry flavor.
Mechanism of Action
Lisdexamfetamine is a prodrug of dextroamphetamine. Amphetamines are non-catecholamine sympathomimetic amines with CNS stimulant activity. The exact mode of therapeutic action in ADHD and BED is not known.
What is the most important information I should know about Vyvanse?
Vyvanse is a federally controlled substance (CII) because it can be abused or lead to dependence. Keep Vyvanse in a safe place to prevent misuse and abuse. Selling or giving away Vyvanse may harm others, and is against the law.
Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
Vyvanse is a stimulant medicine. Some people have had the following problems when taking stimulant medicines such as Vyvanse:
1. Heart-related problems including:
- sudden death in people who have heart problems or heart defects
- sudden death, stroke and heart attack in adults
- increased blood pressure and heart rate
Tell your doctor if you have any heart problems, heart defects, high blood pressure, or a family history of these problems.
Your doctor should check you carefully for heart problems before starting Vyvanse.
Your doctor should check your blood pressure and heart rate regularly during treatment with Vyvanse.
Call your doctor right away if you have any signs of heart problems such as chest pain, shortness of breath, or fainting while taking Vyvanse.
2. Mental (psychiatric) problems including:
- In children, teenagers, and adults
- new or worse behavior and thought problems
- new or worse bipolar illness
- In children and teenagers
- new psychotic symptoms such as:
- hearing voices
- believing things that are not true
- being suspicious
- new manic symptoms
- new psychotic symptoms such as:
Tell your doctor about any mental problems you have or if you have a family history of suicide, bipolar illness, or depression.
Call your doctor right away if you have any new or worsening mental symptoms or problems while taking Vyvanse, especially:
- seeing or hearing things that are not real
- believing things that are not real
- being suspicious
3. Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud’s phenomenon]:
- Fingers or toes may feel numb, cool, painful
- Fingers or toes may change color from pale, to blue, to red
Tell your doctor if you have numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes.
Call your doctor right away if you have any signs of unexplained wounds appearing on fingers or toes while taking Vyvanse.
Who should not take Vyvanse?
Do not take Vyvanse if you:
- are taking or have taken within the past 14 days an anti-depression medicine called a monoamine oxidase inhibitor or MAOI.
- are sensitive to, allergic to, or had a reaction to other stimulant medicines.
What should I tell my healthcare provider before taking Vyvanse?
Before you take Vyvanse, tell your doctor if you have or if there is a family history of:
- heart problems, heart defects, high blood pressure
- mental problems including psychosis, mania, bipolar illness, or depression
- circulation problems in fingers and toes
Also tell your doctor if:
- you have any kidney problems. Your doctor may lower your dose.
- you are pregnant or plan to become pregnant. It is not known if Vyvanse may harm your unborn baby.
- you are breastfeeding or plan to breastfeed. Vyvanse can pass into your milk. Do not breastfeed while taking Vyvanse. Talk to your doctor about the best way to feed your baby if you take Vyvanse.
Tell your doctor about all of the medicines that you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Vyvanse can affect the way other medicines work, and other medicines may affect how Vyvanse works. Using Vyvanse with other medicines can cause serious side effects.
Especially tell your doctor if you take anti-depression medicines including MAOIs.
Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Know the medicines that you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
Do not start any new medicine while taking Vyvanse without talking to your doctor first.
How should I take Vyvanse?
- Take Vyvanse exactly as your doctor tells you to take it.
- Your doctor may change your dose until it is right for you.
- Take Vyvanse 1 time each day in the morning.
- Vyvanse can be taken with or without food.
- Vyvanse comes in capsules or chewable tablets.
Capsules:
- Vyvanse capsules may be swallowed whole.
- If you have trouble swallowing capsules, open your Vyvanse capsule and pour all the powder into yogurt, water, or orange juice.
- Use all of the Vyvanse powder from the capsule so you get all of the medicine.
- Using a spoon, break apart any powder that is stuck together. Stir the Vyvanse powder and yogurt, water or orange juice until they are completely mixed together.
- Eat all the yogurt or drink all the water or orange juice right away after it has been mixed with Vyvanse. Do not store the yogurt, water, or orange juice after it has been mixed with Vyvanse. It is normal to see a filmy coating on the inside of your glass or container after you eat or drink all the Vyvanse.
Chewable Tablets:
- Vyvanse chewable tablets must be completely chewed before swallowing.
Your doctor may sometimes stop Vyvanse treatment for a while to check your ADHD or your BED symptoms.
Your doctor may do regular checks of your heart, and blood pressure while taking Vyvanse.
Children should have their height and weight checked often while taking Vyvanse. Vyvanse treatment may be stopped if a problem is found during these check-ups.
If you take too much Vyvanse, call your doctor or poison control center (1-800-222-1222) right away, or get to the nearest hospital emergency room.
What should I avoid while taking Vyvanse?
Do not drive, operate machinery, or do other dangerous activities until you know how Vyvanse affects you.
What are the possible side effects of Vyvanse?
Vyvanse may cause serious side effects, including:
- See “What is the most important information I should know about Vyvanse?”
- slowing of growth (height and weight) in children
The most common side effects of Vyvanse in ADHD include:
- anxiety
- dry mouth
- trouble sleeping
- decreased appetite
- irritability
- upper stomach pain
- diarrhea
- loss of appetite
- vomiting
- dizziness
- nausea
- weight loss
The most common side effects of Vyvanse in BED include:
- dry mouth
- constipation
- trouble sleeping
- feeling jittery
- decreased appetite
- anxiety
- increased heart rate
Talk to your doctor if you have any side effects that bother you or do not go away.
These are not all the possible side effects of Vyvanse. For more information ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Vyvanse.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Vyvanse for a condition for which it was not prescribed. Do not give Vyvanse to other people, even if they have the same symptoms that you have. It may harm them and it is against the law.
If you would like more information, talk with your doctor. You can ask your pharmacist or healthcare provider for information about Vyvanse that is written for health professionals.
How should I store Vyvanse?
- Store Vyvanse at room temperature, 68°F to 77°F (20°C to 25°C).
- Protect Vyvanse from light.
- Store Vyvanse in a safe place, like a locked cabinet.
- Do not throw away unused Vyvanse in your household trash as it may harm other people or animals. Ask your doctor or pharmacist about a medicine take-back program in your community.
Keep Vyvanse and all medicines out of the reach of children.
What are the ingredients in Vyvanse?
Active ingredient: lisdexamfetamine dimesylate
Inactive ingredients:
Capsule: microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. The capsule shells (imprinted with S489) contain gelatin, titanium dioxide, and one or more of the following: C Red #3, C Yellow #6, C Blue #1, Black Iron Oxide, and Yellow Iron Oxide.
Chewable tablet: colloidal silicon dioxide, croscarmellose sodium, guar gum, magnesium stearate, mannitol, microcrystalline cellulose, sucralose, artificial strawberry flavor.
Label
PRINCIPAL DISPLAY PANEL – 10 MG CAPSULE BOTTLE LABEL
- NDC 59417-101-10
- Vyvanse®
(lisdexamfetamine
dimesylate) capsules - 10 mg
- CII
Rx only - 100 CAPSULES
Takeda


PRINCIPAL DISPLAY PANEL – 20 MG CAPSULE BOTTLE LABEL
- NDC 59417-102-10
- Vyvanse®
(lisdexamfetamine
dimesylate) capsules - 20 mg
- CII
Rx only - 100 CAPSULES
Takeda

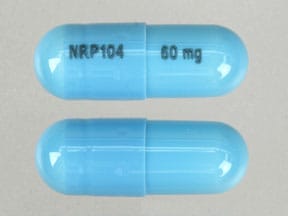
PRINCIPAL DISPLAY PANEL – 60 MG CAPSULE BOTTLE LABEL
- NDC 59417-106-10
- Vyvanse®
(lisdexamfetamine
dimesylate) capsules - 60 mg
- CII
Rx only - 100 CAPSULES
Takeda

SRC: NLM .